Filed Pursuant to Rule 424(b)(5)
Registration No. 333-264190
PROSPECTUS SUPPLEMENT
(To Prospectus dated April 13, 2022)

Up to $23,000,000
Ordinary Shares
We have entered into an Open Market Sale AgreementSM, or the Sale Agreement,
with Jefferies LLC, or Jefferies, dated April 19, 2022, relating to our ordinary shares, par value NIS 0.1 per share, offered by this prospectus supplement and the accompanying prospectus. Under this prospectus supplement we may offer and sell our
ordinary shares having an aggregate offering price of up to $23,000,000 from time to time through Jefferies, acting as our agent.
Sales of our ordinary shares, if any, under this prospectus supplement and the accompanying prospectus may be made by any method permitted that is deemed an
“at the market” offering as defined in Rule 415(a)(4) promulgated under the Securities Act of 1933, as amended, or the Securities Act, including sales made directly on or through the Nasdaq Global Market, the existing trading market for our ordinary
shares. Jefferies is not required to sell any specific number or dollar amount of securities but will act as our sales agent using commercially reasonable efforts consistent with its normal trading and sales practices, on mutually agreed terms
between Jefferies and us. There is no arrangement for funds to be received in any escrow, trust or similar arrangement.
Jefferies will be entitled to compensation under the terms of the Sale Agreement at a commission rate of 3.0% of the gross sales price per share sold. See
“Plan of Distribution” beginning on page S-24 for additional information regarding the compensation to be paid to Jefferies. In connection with the sale of our ordinary shares on our behalf, Jefferies will be deemed to be an “underwriter” within the
meaning of the Securities Act and the compensation of Jefferies will be deemed to be underwriting commissions or discounts. We have also agreed to provide indemnification and contribution to Jefferies with respect to certain liabilities, including
civil liabilities under the Securities Act.
Our ordinary shares are traded on the Nasdaq Global Market under the symbol “SLGL.” On April 14, 2022, the last reported sale price of our ordinary shares
was $7.19 per share.
As of April 14, 2022, the aggregate market value worldwide of our outstanding voting and non-voting common equity held by non-affiliates
was approximately $70.7 million, based on 23,126,804 ordinary shares outstanding, of which 8,972,240 ordinary shares were held by non-affiliates, and a per ordinary share price of $7.88 based on the closing sale price of our ordinary shares on the
Nasdaq Global Market on March 30, 2022. Pursuant to General Instruction I.B.5 of Form F-3, in no event will we sell, pursuant to the registration statement of which this prospectus supplement forms a part, securities with a value exceeding one-third
of the aggregate market value of our outstanding ordinary shares held by non-affiliates in any 12-month period, so long as the aggregate market value of our ordinary shares held by non-affiliates is less than $75.0 million. We have not offered or
sold any securities pursuant to General Instruction I.B.5 on Form F-3 during the prior 12 calendar month period that ends on and includes the date of this prospectus.
We are an “emerging growth company” as that term is used in the Jumpstart Our Business Startups Act of 2012 and, as such, have elected to comply with certain
reduced public company reporting requirements for this prospectus and future filings.
Investing in our ordinary shares involves a high degree of risk. Before making an investment decision, you should carefully consider all of the information
set forth in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein. You should review carefully the risks and uncertainties described under the heading “Risk Factors” beginning on page
S-7 of this prospectus supplement and under similar headings in the other documents that are incorporated by reference into this prospectus supplement and accompanying prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of
these securities or determined if this prospectus supplement and the accompanying prospectus are truthful or complete. Any representation to the contrary is a criminal offense.
Jefferies
The date of this prospectus supplement is April 19, 2022.
PROSPECTUS SUPPLEMENT
Page
|
S-ii
|
|
|
S-iii
|
|
|
S-1
|
|
|
S-7
|
|
|
S-9
|
|
|
S-10
|
|
|
S-11
|
|
|
S-12
|
|
|
S-13
|
|
|
S-23
|
|
|
S-25
|
|
|
S-25
|
|
|
S-25
|
|
|
S-25
|
|
|
S-26
|
PROSPECTUS
Page
| 1 |
|
| 2 |
|
| 3 |
|
| 3 |
|
| 3 |
|
| 4 |
|
| 4 |
|
| 4 |
|
| 5 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 10 |
|
| 10 |
|
| 10 |
|
| 11 |
|
| 11 |
S - i
This prospectus supplement and the accompanying prospectus are part of a “shelf” registration statement on Form F-3 (File No.
333-264190) that we initially filed with the Securities and Exchange Commission on April 7, 2022, and that was declared effective by the SEC on April 13, 2022. This document is in two parts. The first part is this prospectus supplement, which
describes the terms of this offering of our ordinary shares and adds to and updates the information contained in the accompanying prospectus and the documents incorporated or deemed to be incorporated by reference in this prospectus supplement and
the accompanying prospectus. The second part, the accompanying prospectus, provides more general information, some of which may not apply to this offering. Generally, when we refer to this prospectus, we are referring to both parts of this document
combined. If the description of the offering varies between this prospectus supplement and the accompanying prospectus or the documents incorporated by reference herein or therein filed prior to the date of this prospectus supplement, you should rely
on the information contained in this prospectus supplement. However, if any statement in one of these documents is inconsistent with a statement in another document having a later date — for example, a document incorporated by reference in the
accompanying prospectus — the statement in the document having the later date modifies or supersedes the earlier statement.
This prospectus supplement and the accompanying prospectus relate to the offering of our ordinary shares. Before buying any of the
ordinary shares offered hereby, we urge you to read carefully this prospectus supplement and the accompanying prospectus, together with the information incorporated herein by reference as described below under the heading “Incorporation of Certain
Documents by Reference.” This prospectus supplement contains information about the ordinary shares offered hereby and may add to, update or change information in the accompanying prospectus.
You should rely only on the information contained in, or incorporated by reference into, this prospectus supplement and the accompanying
prospectus. We have not, and Jefferies has not, authorized anyone to provide you with different or additional information.
We are not making offers to sell or solicitations to buy our ordinary shares in any jurisdiction in which an offer or solicitation is
not authorized or in which the person making that offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to make an offer or solicitation. You should assume that the information in this prospectus supplement and the
accompanying prospectus is accurate only as of the date on the front of the respective document and that any information that we have incorporated by reference is accurate only as of the date of the document incorporated by reference in this
prospectus supplement or the accompanying prospectus, regardless of the time of delivery of this prospectus supplement or the accompanying prospectus or the time of any sale of a security.
This prospectus supplement and the accompanying prospectus contain summaries of certain provisions contained in some of the documents
described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed
or will be incorporated in this prospectus supplement and the accompanying prospectus by reference as exhibits to the registration statement, and you may obtain copies of those documents as described below under the section entitled “Where You Can
Find More Information.”
We further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any
document that is incorporated by reference in this prospectus supplement and the accompanying prospectus were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties
to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and
covenants should not be relied on as accurately representing the current state of our affairs.
Solely for convenience, the trademarks, service marks and trade names referred to or
incorporated by reference in this prospectus supplement and the accompanying prospectus are without the ® and ™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable
law, our rights or the rights of the applicable licensors to these trademarks, service marks and trade names. This prospectus supplement and the accompanying prospectus contain additional trademarks, service marks and trade names of others, which
are the property of their respective owners. All trademarks, service marks and trade names appearing in this prospectus supplement and the accompanying prospectus are, to our knowledge, the property of their respective owners. We do not intend our
use or display of other companies’ trademarks, service marks or trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
Unless otherwise mentioned or unless the context requires otherwise, all references in this prospectus supplement and the accompanying
prospectus to “Sol-Gel,” “Sol-Gel Technologies,” “we,” “us,” “our,” “the Company” and similar designations refer to Sol-Gel Technologies Ltd. and its wholly-owned subsidiary, Sol-Gel Technologies, Inc.
S - ii
This prospectus supplement and the accompanying prospectus contain and incorporate by reference statistics and other data relating to
markets, market sizes and other industry data pertaining to our business that we have obtained from industry publications and surveys and other information available to us. Industry publications and surveys generally state that the information
contained therein has been obtained from sources believed to be reliable. Market data and statistics are inherently predictive and speculative and are not necessarily reflective of actual market conditions. Such statistics are based on market
research, which itself is based on sampling and subjective judgments by both the researchers and the respondents, including judgments about what types of products and transactions should be included in the relevant market. In addition, the value of
comparisons of statistics for different markets is limited by many factors, including that (i) the markets are defined differently, (ii) the underlying information was gathered by different methods, and (iii) different assumptions were applied in
compiling the data. Accordingly, the market statistics included or incorporated by reference in this prospectus supplement and the accompanying prospectus should be viewed with caution. We believe that information from these industry publications
included in this prospectus supplement and the accompanying prospectus is reliable.
S - iii
This summary highlights certain information about us, this offering and selected information contained elsewhere in
or incorporated by reference into this prospectus supplement and the accompanying prospectus. This summary is not complete and does not contain all of the information that you should consider before deciding whether to invest in our ordinary shares.
For a more complete understanding of our company and this offering, we encourage you to read and consider carefully the more detailed information in this prospectus supplement and the accompanying prospectus, including the information incorporated by
reference in this prospectus supplement and the accompanying prospectus, and the information included in any free writing prospectus that we have authorized for use in connection with this offering, including the information under the heading “Risk
Factors” in this prospectus supplement on page S-7 and in the documents incorporated by reference into this prospectus supplement and the accompanying prospectus.
Our Company
We are a dermatology company focused on identifying, developing and commercializing investigational and generic
topical drug products for the treatment of skin diseases. In addition to Twyneo®, which has been approved by the FDA, our current product candidate pipeline consists of
clinical stage and early-stage investigational product candidates, some of which leverage our development platform, and several generic product candidates across multiple indications.
Our FDA-approved product, Twyneo®,
is a novel, once-daily, non-antibiotic topical cream containing a fixed-dose combination of encapsulated benzoyl peroxide and encapsulated tretinoin, that we developed for the treatment of acne vulgaris, or acne.
Our investigational product candidate, Epsolay®, is a novel, once-daily investigational topical cream containing
encapsulated benzoyl peroxide, that we are developing for the treatment of papulopustular (subtype II) rosacea.
In June 2021, we entered into two five-year exclusive license agreements with Galderma Holding SA (“Galderma”) pursuant to which Galderma has
the exclusive right to, and is responsible for, all U.S. commercial activities for Twyneo®, and, if approved by the FDA, Epsolay®.
Other investigational product candidates are SGT-210 that we are developing for the treatment of various
keratodermas; SGT-310, an investigational aryl hydrocarbon receptor agonist; and SGT-510.
We designed our proprietary, silica-based microencapsulation technology platform to enhance the tolerability and
stability of topical drugs while maintaining their efficacy. Topical drugs often struggle to balance achieving both high efficacy and high tolerability. Our technology platform entraps active ingredients in an inert, inorganic silica shell, which
creates an unnoticeable barrier between the active ingredient and the skin. The resulting microcapsules are designed to allow the entrapped active ingredients to gradually migrate through the pores of the shell and deliver active ingredient doses
onto the skin in a controlled manner, resulting in improved tolerability and stability without sacrificing efficacy. By separately encapsulating active ingredients within protective silica shells, our technology platform also enables the production
of novel fixed-dose active ingredient combinations that otherwise would not be stable. We believe that our microencapsulation technology has the potential to be used for topical drug products to treat a variety of skin diseases. As a result of the
FDA having already approved silica as a safe excipient for topical drug products, Both Twyneo® and Epsolay® were submitted for approval through the FDA’s 505(b)(2) regulatory pathway.
Product Pipeline
The following chart represents our current branded and generic product candidate pipeline.
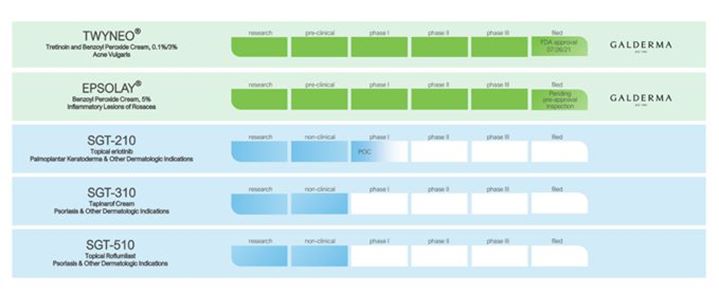
S - 1
Our Branded Product Candidates
Our Approved Product and Investigational Product Candidates
Twyneo® for Acne
Using our proprietary, silica-based microencapsulation technology platform, we developed Twyneo® to become a preferred treatment for acne by dermatologists and their patients.
Twyneo® is a novel, once-daily,
non-antibiotic topical cream containing a fixed-dose combination of encapsulated benzoyl peroxide and encapsulated tretinoin that we developed for the treatment of acne. Studies have shown that benzoyl peroxide and tretinoin are effective in treating
acne as monotherapies; moreover, according to an article in the American Academy of Dermatology (2009), dermatologists recommend combining the two monotherapies as a first-line approach for acne, but a drug-drug interaction that causes the
degradation of tretinoin has previously prohibited the development of a combination therapy. By encapsulating the two agents separately through the use of our technology platform, Twyneo® is designed to be a fixed-dose combination that otherwise would not be stable. Similar to other combination drug products, such as clindamycin and benzoyl peroxide, Twyneo® is required to be kept refrigerated throughout the supply chain and then stored in ambient conditions upon its distribution to patients. Pre-clinical data suggests that Twyneo® may be more tolerable than generic tretinoin gel 0.1% and Epiduo, a branded fixed-dose combination of benzoyl peroxide and adapalene, without a corresponding loss in efficacy. In addition, Epiduo and its
successor Epiduo Forte contain adapalene as opposed to tretinoin, which is widely considered to be more effective than adapalene, but generally causes greater irritation. We expect that Twyneo® will compete directly with Winlevi, Aklief, Epiduo and
Epiduo Forte. We have utilized the FDA’s 505(b)(2) regulatory pathway in seeking approval of Twyneo® in the United States.
On December 30, 2019, we announced top-line results from two pivotal Phase 3 clinical trials evaluating Twyneo® for the treatment of acne. Twyneo® met all co-primary endpoints in both Phase 3
trials. The Phase 3 program enrolled an aggregate of 858 patients aged nine and older in two multicenter, randomized, double-blind, parallel group, vehicle-controlled trials at 63 sites across the United States. Twyneo® demonstrated statistically significant improvement in each of the co-primary endpoints of (1) the proportion of patients who succeeded in achieving at least a two grade reduction from baseline
and Clear (grade 0) or Almost Clear (grade 1) at Week 12 on a 5-point Investigator Global Assessment (IGA) scale, (2) an absolute change from baseline in inflammatory lesion count at Week 12, and (3) and an absolute change from baseline in
non-inflammatory lesion count at Week 12. Twyneo® was approved for marketing by the FDA in July 2021.
S - 2
Epsolay® for Subtype II Rosacea
Epsolay® Overview
Epsolay® is a once-daily investigational topical cream containing 5% encapsulated benzoyl peroxide that we have
developed for the treatment of papulopustular (subtype II) rosacea. We believe Epsolay® has the potential to become the first product to contain encapsulated benzoyl peroxide for the treatment of subtype II rosacea and, if approved by the FDA, has
the potential to redefine the standard of care for the treatment of inflammatory lesions associated with subtype II rosacea. Subtype II rosacea is characterized by small, dome-shaped erythematous papules, tiny surmounting pustules on the central
aspects of the face, solid facial erythema and edema, and thickening/overgrowth of skin. Subtype II rosacea resembles acne, except that comedones are absent, and patients may report associated burning and stinging sensations. We expect that Epsolay®,
if approved by the FDA, will compete directly with Soolantra. We utilized the FDA’s 505(b)(2) regulatory pathway in seeking approval of Epsolay® in the United States. On July 8, 2019, we announced positive
top-line results from our Phase 3 program evaluating Epsolay®. The program enrolled 733 patients aged 18 and older in two identical, double-blind, vehicle-controlled Phase 3 clinical trials at 54 sites across the United States. Epsolay® demonstrated
statistically significant improvement in both co-primary endpoints of (1) the number of patients achieving “clear” or “almost clear” in the Investigator Global Assessment (IGA) relative to baseline at week 12 and (2) absolute mean reduction from
baseline in inflammatory lesion count at week 12. In an additional analysis, Epsolay® demonstrated rapid efficacy, achieving statistically significant improvements on both co-primary endpoints compared with vehicle as early as Week 2. In addition,
Epsolay® was found to be well- tolerated. On February 12, 2020, we announced positive topline results from our open-label, long-term safety study, evaluating Epsolay® for a treatment duration up to 52 weeks. Our NDA for Epsolay® has been accepted for filing by the FDA, which originally assigned a PDUFA goal date of April 26, 2021, which has since been delayed due to COVID-19 related travel
restrictions. The FDA conducted a pre-approval inspection of the production site for Epsolay® during the week of February 14, 2022.
Our Solution for Subtype II Rosacea — Epsolay®
Benzoyl peroxide is approved by the FDA for the treatment of acne and is widely considered to be safe and
effective. Currently, there is no approved benzoyl peroxide product in the rosacea treatment landscape as a result of potential tolerability issues, despite clinical studies showing that treatment with benzoyl peroxide could be efficacious. According
to a published study, benzoyl peroxide was found to be an effective treatment for rosacea but caused irritation. Using our proprietary, silica-based microencapsulation technology platform, we believe our Epsolay® candidate for the treatment of
papulopustular (subtype II) rosacea can improve on current subtype II rosacea treatments in the following ways:
| • |
Epsolay® creates a silica-based barrier between benzoyl peroxide crystals and the skin and, as a result, can reduce irritation typically associated with topical application of benzoyl peroxide, increasing the potential for more tolerable
application to rosacea-affected skin.
|
| • |
Epsolay®'s release of the drug can reduce irritation while maintaining efficacy.
|
Epsolay® is an innovative topical cream, and if approved by the FDA, would be the first product containing benzoyl
peroxide for the treatment of subtype II rosacea.
SGT-210 for Keratodermas
We are developing SGT-210 for the treatment of keratoderma, such as palmoplantar keratoderma (“PPK”), a group of skin conditions characterized
by thickening of the skin. SGT-210 is designed to be used alone or in combination for the treatment of hyperproliferation and hyperkeratinization disorders, including PPK. On January 2, 2020, we announced the initiation of a Phase 1 clinical study
of SGT-210 in patients with palmoplantar keratoderma. The Phase 1 concept study SGT-84-01 is a single-center, single-blinded, vehicle-controlled study designed to evaluate the bioavailability, safety and tolerability of SGT-210 as well as inform on
potential efficacy. During the third quarter of 2021, we reported that the study with respect to six PPK patients has been completed and indicated modest improvement and a favorable safety profile.
SGT-210, SGT-310 and SGT-510 potentially for psoriasis and other medical conditions
We are conducting pre-clinical testing to explore the possible activity of SGT-210, SGT-310, and SGT-510 in various new pharmaceutical indications. Approximately 25 provisional
patent applications for these project candidates have been submitted to date, including patent applications covering the use of tapinarof in ophthalmic disorders such as dry eye, uveitis, and blepharitis with or without demodex involvement.
S - 3
Generic Drug Product Candidates
In addition to our investigational product candidates, we are also currently developing a portfolio of two generic topical dermatological
products related to four generic drug candidates in collaboration with Padagis by assignment from Perrigo UK Finco Limited Partnership (“Perrigo”). Padagis has significant experience in the development of generic drugs.
We previously had collaboration arrangements with Perrigo to develop a portfolio of 11 generic topical
dermatological products. In November 2021, we announced that we had signed an agreement with Padagis, pursuant to which we sold our rights related to 10 generic collaborative agreements between the parties. Under the terms of this agreement with
Padagis, effective as of November 1, 2021, we are to unconditionally receive $21.5 million over 24 months, in lieu of our share in the ten generic programs, two of which were approved by the FDA, and eight of which are unapproved. Pursuant to the
agreement, effective as of November 1, 2021, we ceased paying any outstanding and future operational costs related to these 10 collaborative agreements.
We currently have two collaboration agreements with Padagis for the development, manufacturing and
commercialization of two generic product candidates. Under such agreements, Padagis will conduct the regulatory (if relevant), scientific, clinical and technical activities necessary to develop the generic product candidates and seek regulatory
approval with the FDA for the generic product candidates. If approved by the FDA, Padagis has agreed to commercialize the generic product candidates in the United States. We and Padagis will share the development costs and the gross profits generated
from the sales of the generic product candidates, if approved by the FDA.
Commercialization Strategy
We currently have limited sales, marketing and distribution capabilities. In June 2021, we entered into two
five-year exclusive license agreements with Galderma pursuant to which Galderma has the exclusive right to, and is responsible for, all U.S. commercial activities for Twyneo®, and, if approved by the FDA, Epsolay®. Pursuant to the agreement, we are
entitled to consideration of up to $11 million in upfront payments to us and regulatory approval milestone payments. We are also eligible to receive tiered double-digit royalties ranging from mid-teen to high-teen percentage of net sales as well as
up to $9 million in sales milestone payments. We also expect to collaborate with third parties that have sales and marketing experience in order to commercialize our other investigational product candidates, if approved by the FDA for commercial
sale, in lieu of our own sales force and distribution systems. If we are unable to enter into such arrangements for our other product candidates on acceptable terms or at all, we may not be able to successfully commercialize them. In other markets,
we also expect to selectively pursue strategic collaborations with third parties in order to maximize the commercial potential of our product candidates.
Corporate Information
Our legal and commercial name is Sol-Gel Technologies Ltd. We were incorporated on October 28, 1997 and were registered as a company
with limited liability under the laws of the State of Israel.
Our principal executive offices are located at 7 Golda Meir St., Weizmann Science Park, Ness Ziona, 7403650 Israel, and our telephone
number is +972-8-931-3433. Our website address is http://www.sol-gel.com. The information on our website does not constitute a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
Our agent for service of process in the United States is Sol-Gel Technologies, Inc., c/o The Corporation Trust Company, located at Corporation Trust Center, 1209 Orange Street, Wilmington, Delaware 19801.
Implications of Being an Emerging Growth Company and a Foreign Private Issuer
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, and therefore we may take advantage
of certain exemptions from various public company reporting requirements, including not being required to have our internal control over financial reporting audited by our independent registered public accounting firm pursuant to Section 404 of the
Sarbanes Oxley Act of 2002, or the Sarbanes-Oxley Act, and reduced financial reporting requirements. We may take advantage of these exemptions until we are no longer an “emerging growth company.” We will remain an emerging growth company until the
earliest of: (1) the end of the fiscal year in which the market value of our ordinary shares that are held by non-affiliates is at least $700 million as of the last business day of our most recently completed second fiscal quarter, (2) the end of the
fiscal year in which we have total annual gross revenues of $1.07 billion or more during such fiscal year, (3) the date on which we have issued more than $1 billion in non-convertible debt in a three-year period, and (4) the last day of the fiscal
year following the fifth anniversary of our initial public offering, which was completed in February 2018.
S - 4
Even after we no longer qualify as an emerging growth company, as long as we qualify as a foreign private issuer under the Securities
Exchange Act of 1934, or the Exchange Act, we will be exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including:
| • |
the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act;
|
| • |
the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and
|
| • |
the rules under the Exchange Act requiring filing with the SEC of quarterly periodic reports on Form 10-Q containing unaudited financial and other specific information, or current reports on Form 8-K, upon the
occurrence of specified significant events.
|
Both foreign private issuers and emerging growth companies also are exempt from certain more stringent executive compensation disclosure
rules. Thus, even if we no longer qualify as an emerging growth company, but remain a foreign private issuer, we will continue to be exempt from the more stringent compensation disclosures required of companies that are neither an emerging growth
company nor a foreign private issuer.
S - 5
THE OFFERING
|
Ordinary shares offered by us
|
Our ordinary shares, par value NIS 0.1 per share, having an aggregate offering price of up to $23,000,000.
|
|
|
|
|
Manner of offering
|
“At the market” offering that may be made from time to time through our sales agent, Jefferies LLC. See “Plan of Distribution.”
|
|
|
|
|
Use of Proceeds
|
We intend to use the net proceeds from this offering to fund research and development activities and the remainder for working capital
and other general corporate purposes. See “Use of Proceeds.”
|
|
|
|
|
Risk Factors
|
Investing in our ordinary shares involves significant risks. See “Risk Factors” on page S-7 of this prospectus supplement, and under similar headings in other documents incorporated by reference into this
prospectus supplement and the accompanying prospectus.
|
|
|
|
|
Nasdaq Global Market symbol
|
“SLGL”
|
S - 6
An investment in our securities involves a high degree of risk. Our business, financial condition or results of operations could be
adversely affected by any of these risks. You should carefully consider the risk factors discussed below and the risk factors under the caption “Item 3: Key Information—D. Risk Factors” in our Annual Report on Form 20-F for the year ended December
31, 2021, and in any other filing we make with the SEC subsequent to the date of this prospectus supplement that is incorporated herein by reference, before making your investment decision. The risks and uncertainties we have described are not the
only ones we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our operations. Past financial performance may not be a reliable indicator of future performance, and historical
trends should not be used to anticipate results or trends in future periods. If any of these risks actually occurs, our business, business prospects, financial condition or results of operations could be seriously harmed. This could cause the
trading price of our ordinary shares to decline, resulting in a loss of all or part of your investment. Please also read carefully the section below entitled “Forward-Looking Statements.”
Risks Related to the Offering and Our Ordinary Shares
Our management and board of directors will have broad discretion as to the use of the net proceeds from this
offering, and we may not use them effectively.
We intend to use the net proceeds from this offering to fund research and development activities and the remainder for working capital and
other general corporate purposes. However, our management and board of directors will have broad discretion in the application of the net proceeds from this offering, and you will be relying on their judgment regarding the application of these
proceeds, which can be different from that contemplated at the time of this offering. Our management and board of directors could spend the proceeds in ways that do not improve our results of operations or enhance the value of our ordinary
shares. Our failure to apply these funds effectively could have a material adverse effect on our business, delay the development of our products and cause the price of our ordinary shares to decline.
You will experience immediate and substantial dilution in the book value per ordinary share you purchase.
The price per share in this offering may exceed the net tangible book value per share of our ordinary shares outstanding prior to this offering. Assuming that an aggregate of 3,198,887 ordinary
shares are sold during the term of the Sale Agreement with Jefferies LLC, or Jefferies, at a price of $7.19 per share (the last reported sale price of our ordinary shares on the Nasdaq Global Market on April 14, 2022), for aggregate gross proceeds
of approximately $23,000,000, and after deducting commissions and estimated offering expenses payable by us, you will experience immediate dilution of $4.23 per share, representing the difference between the assumed offering price per share and our
as adjusted net tangible book value per share as of December 31, 2021 after giving effect to this offering. If holders of outstanding options to acquire our ordinary shares exercise those options at prices below the public offering price per share,
and upon vesting of outstanding restricted share units that we have granted, you will experience further dilution. See the section titled “Dilution” below for a more detailed illustration of the dilution you would incur if you participate in this
offering.
A large number of shares may be sold in the market following this offering, which may depress the market price of
our ordinary shares.
All of our ordinary shares sold in this offering will be freely tradable without restriction or further registration under the Securities Act. As a result, a substantial number
of our ordinary shares may be sold in the public market following this offering, which may cause the market price of our ordinary shares to decline. This could make it more difficult for you to sell your ordinary shares at a time and price that you
deem appropriate and could impair our ability to raise capital through the sale of additional equity securities.
You may experience further dilution as a result of future equity offerings.
To raise additional capital, we may in the future offer additional ordinary shares or other securities convertible into or exchangeable for our ordinary shares at prices that may
not be the same as the price per share in this offering. We may sell ordinary shares or other securities in any other offering at a price per share that is less than the price per share paid by investors in this offering, and investors purchasing
shares or other securities in the future could have rights superior to existing shareholders. The price per share at which we sell additional ordinary shares, or securities convertible or exchangeable into ordinary shares, in future transactions
may be higher or lower than the price per share paid by investors in this offering.
S - 7
The actual number of ordinary shares we will issue under the Sale Agreement, at any one time or in total, is uncertain.
Subject to certain limitations in the Sale Agreement and compliance with applicable law, we have the discretion to deliver an issuance notice to Jefferies at any time during the term of the Sale
Agreement. Because the number of shares that are sold by Jefferies after delivery of the issuance notice to Jefferies will fluctuate based on the market price of the ordinary shares during the sales period and any limits we set with Jefferies, it
is not possible at this stage to predict the number of ordinary shares that will ultimately be issued.
We may be considered to be a passive foreign investment company for U.S. federal income tax purposes for the current
tax year and possibly thereafter, which could result in materially adverse U.S. federal income tax consequences to U.S. Holders of our ordinary shares.
A non-U.S. entity treated as a corporation for U.S. federal income tax purposes will be a passive foreign investment
company, or PFIC, for any taxable year if either (i) at least 75% of its gross income for such year is passive income or (ii) at least 50% of the value of its assets (based on an average of the quarterly values of the assets) during such year is
attributable to assets that produce passive income or are held for the production of passive income. For our 2019 through 2021 taxable years we generated revenue under our then collaboration agreement with Perrigo for the development of a generic
product candidate. In 2021, we sold our rights to this and other generic products and will unconditionally receive further revenue over 24 months in lieu of our share in the collaboration agreements with respect to these products. Starting in 2021,
we began generating revenue under our license agreements with Galderma for Twyneo®, and Epsolay®. Though the application of the relevant rules governing the characterization of the foregoing revenue for purposes of the PFIC income test is
uncertain, we intend to take the position that, based on our involvement and management contributions throughout the development process, such revenue is non-passive for PFIC purposes. As a result, based on the current and anticipated value and
composition of our income and assets, we do not expect that we will be treated as a PFIC for U.S. federal income tax purposes for our current taxable year or for foreseeable future years. However, there are substantial factual and legal ambiguities
regarding the nature of the revenue and the application of the relevant PFIC rules, and thus, the determination that such revenue is non-passive is not without doubt, and alternative characterizations are possible.
A separate determination has to be made after the close of each taxable year as to whether we were a PFIC for that year. Because the
value of our assets for purposes of the PFIC test will generally be determined by reference to the market price of our ordinary shares, our PFIC status may depend in part on the market price of our ordinary shares, which may fluctuate significantly.
In addition, there are certain other ambiguities in applying the PFIC test to us. If we are considered a PFIC, material adverse U.S. federal income tax consequences could apply to U.S. Holders (as defined in the
section headed “Material Tax Considerations—U.S. Federal Income Tax Considerations”) of our ordinary shares with respect to any “excess distribution” received from us and any gain from a sale or other disposition of our ordinary shares. Potential
investors are strongly advised to consult their own advisors regarding the consequences to them if we were to be considered a PFIC. Please see “Material Tax Considerations—U.S. Federal Income Tax Considerations—Passive Foreign Investment Company”
for more information.
S - 8
We make forward-looking statements in this prospectus supplement, the accompanying prospectus and
the other documents we have filed with the SEC that are incorporated herein and therein by reference, that are subject to risks and uncertainties. These forward-looking statements include information about possible or assumed future results
of our business, financial condition, results of operations, liquidity, plans and objectives. In some cases, you can identify forward-looking statements by terminology such as “believe,” “may,” “estimate,” “continue,” “anticipate,” “intend,”
“should,” “plan,” “expect,” “predict,” “potential,” or the negative of these terms or other similar expressions. Forward-looking statements are based on information we have when these statements are made or our management’s good faith belief as of
that time with respect to future events, and are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in or suggested by the forward-looking statements. Important factors that
could cause such differences include, but are not limited to:
| • |
the adequacy of our financial and other resources, particularly in light of our history of recurring losses and the uncertainty regarding the adequacy of
our liquidity to pursue our complete business objectives;
|
| • |
our ability to complete the development of our investigational product candidates;
|
| • |
our dependance on the success of Galderma in commercializing Twyneo® and Epsolay®;
|
| • |
the possibility that Galderma may terminate the collaboration agreement with respect to Epsolay® since Epsolay® was not approved for marketing by the FDA by March 31, 2022;
|
| • |
our ability to find suitable co-development, contract manufacturing and marketing partners;
|
| • |
our ability to obtain and maintain regulatory approvals for our investigational product candidates in our target markets and the possibility of adverse regulatory or legal actions relating to our
investigational product candidates even if regulatory approval is obtained;
|
| • |
our ability to commercialize and launch our pharmaceutical investigational product candidates;
|
| • |
our ability to obtain and maintain adequate protection of our intellectual property;
|
| • |
our ability to manufacture our investigational product candidates in commercial quantities, at an adequate quality or at an acceptable cost;
|
| • |
acceptance of Twyneo®, Epsolay® and our other investigational product candidates by healthcare professionals and patients;
|
| • |
the possibility that we may face third-party claims of intellectual property infringement;
|
| • |
the timing and results of clinical trials that we may conduct or that our competitors and others may conduct relating to our or their products;
|
| • |
intense competition in our industry, with competitors having substantially greater financial, technological, research and development, regulatory and clinical, manufacturing, marketing and sales, distribution
and personnel resources than we do;
|
| • |
potential product liability claims;
|
| • |
potential adverse federal, state and local government regulation in the United States, Europe or Israel;
|
| • |
the impact of ongoing pandemics such as Novel Coronavirus Disease 2019, or COVID-19, on our business and financial condition; and
|
| • |
loss or retirement of key executives and research scientists.
|
You should review carefully the risks and uncertainties described under the heading “Risk Factors” in this prospectus supplement and in the accompanying prospectus, and under the
heading “Risk Factors” in our most recent Annual Report on Form 20-F and in our other filings with the SEC that are incorporated by reference in this prospectus supplement and the accompanying prospectus, for a discussion of these and other risks
that relate to our business and investing in our ordinary shares. The forward-looking statements contained in this prospectus supplement, the accompanying prospectus and the other documents we have filed with the SEC that are incorporated herein
and therein by reference are expressly qualified in their entirety by this cautionary statement. Except as required by law, we undertake no obligation to update publicly any forward-looking statements after the date of this prospectus supplement to
conform these statements to actual results or to changes in our expectations.
S - 9
We may issue and sell our ordinary shares having aggregate sales proceeds of up to $23,000,000 from time to time. The amount of proceeds from this offering will depend upon the number of ordinary
shares sold and the market price at which they are sold. Because there is no minimum offering amount required as a condition of this offering, the actual total public offering amount, commissions and proceeds to us, if any, are not determinable at
this time. There can be no assurance that we will be able to sell any shares under or fully utilize the Sale Agreement with Jefferies as a source of financing.
We intend to use the net proceeds from the sale of securities offered under this prospectus supplement to fund research and development
activities and the remainder for working capital and other general corporate purposes.
Although we have identified some potential uses of the net proceeds to be received upon completion of this offering, we cannot specify these uses with certainty. Our expected use
of net proceeds from this offering represents our intentions based on our present plans and business conditions, which could change as our plans and business conditions evolve. The amounts and timing of our actual expenditures may vary
significantly depending on numerous factors, including the progress of our product candidate development, the status of, and results from, clinical trials, as well as any collaborations that we have entered into or may enter into with third parties
for our product candidates, and any unforeseen cash needs. As a result, our management and board of directors will have broad discretion in the application of the net proceeds from this offering and could use them for purposes other than those
contemplated at the time of this offering. Our shareholders may not agree with the manner in which our management chooses to allocate and spend the net proceeds. Moreover, our management may use the net proceeds for corporate purposes that may not
result in our being profitable or increase our market value.
Until we use the net proceeds of this offering, we intend to deploy the funds in either (i) cash and cash equivalents or (ii) short-term, investment grade, interest-bearing
instruments, consistent with our investment policy.
S - 10
The table below sets forth our cash and cash equivalents, bank deposits and marketable securities and capitalization as of December 31,
2021:
| • |
on an actual basis; and
|
| • |
on an as adjusted basis to reflect the sale of an aggregate of 3,198,887 ordinary shares in this offering at an assumed offering price of $7.19 per share (the last
reported sale price of our ordinary shares on the Nasdaq Global Market on April 14, 2022) for aggregate gross proceeds of approximately $23,000,000, after deducting commissions and estimated offering expenses payable by us.
|
The actual and as adjusted data included in the table below is unaudited. The financial data in the following table should be read in
conjunction with our financial statements and notes thereto incorporated by reference herein.
|
|
As of December 31, 2021
|
|||||||
|
|
Actual
|
As Adjusted
|
||||||
|
|
(in thousands)
|
|||||||
|
|
||||||||
|
Cash and cash equivalents, bank deposits and marketable securities
|
$
|
43,242
|
$
|
65,452
|
||||
|
Shareholders’ equity
|
||||||||
|
Ordinary shares, par value NIS 0.1 per share; 50,000,000 shares authorized and 23,126,804 shares issued and outstanding, actual; 50,000,000 shares authorized and 26,325,691 issued and
outstanding, as adjusted
|
$
|
638
|
$
|
737
|
||||
|
Additional paid-in capital
|
233,098
|
255,209
|
||||||
|
Accumulated deficit
|
(178,142
|
)
|
(178,142
|
)
|
||||
|
Total shareholders’ equity
|
55,594
|
77,804
|
||||||
|
Total capitalization
|
$
|
55,594
|
$
|
77,804
|
||||
The above table is based on 23,126,804 ordinary shares issued and outstanding as of December 31, 2021, and excludes the following:
| • |
1,329,604 ordinary shares issuable upon the exercise of options to purchase ordinary shares outstanding under our 2014 Share Incentive Plan as of December
31, 2021, at a weighted average exercise price of $ 5.91 per ordinary share; and
|
| • |
an additional 753,578 ordinary shares reserved for future issuance under our amended and restated 2014 Share Incentive Plan.
|
S - 11
If you invest in our ordinary shares in this offering, your interest will be diluted immediately to the extent of the difference between the public offering
price per share and the as adjusted net tangible book value per share of our ordinary shares after this offering. We calculate net tangible book value per ordinary share by subtracting our liabilities from our tangible assets and dividing the
difference by the number of ordinary shares outstanding at a given date.
Our net tangible book value as of December 31, 2021 was approximately $55.6 million, or $2.40 per share.
After giving effect to the sale of $23,000,000 of our ordinary shares in this offering at an assumed public offering price of $7.19 per share (the last
reported sale price of our ordinary shares on the Nasdaq Global Market on April 14, 2022), and after deducting commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of December 31, 2021 would have been
approximately $77.8 million, or $2.96 per ordinary share. This represents an immediate increase in net tangible book value of $0.56 per share to our existing shareholders and immediate dilution in net tangible book value of $4.23 per share to
investors participating in this offering, as illustrated by the following table:
|
Assumed offering price per ordinary share
|
$
|
7.19
|
||||||
|
Net tangible book value per share as of December 31, 2021
|
$
|
2.40
|
||||||
|
Increase in net tangible book value per share attributable to this offering
|
$
|
0.56
|
||||||
|
As adjusted net tangible book value per share as of December 31, 2021, after giving effect to this offering
|
$
|
2.96
|
||||||
|
Dilution per share to investors participating in this offering
|
$
|
4.23
|
||||||
The table above assumes for illustrative purposes that an aggregate of 3,198,887 of our ordinary shares are sold at a price of $7.19 per share (the last
reported sale price of our ordinary shares on the Nasdaq Global Market on April 14, 2022), for aggregate gross proceeds of approximately $23,000,000. The shares sold in this offering, if any, will be sold from time to time at various prices.
The above table is based on 23,126,804 ordinary shares issued and outstanding as of December 31, 2021, and excludes the following:
| • |
1,329,604 ordinary shares issuable upon the exercise of options to purchase ordinary shares outstanding under our 2014 Share Incentive Plan as of December
31, 2021, at a weighted average exercise price of $ 5.91 per ordinary share; and
|
| • |
an additional 753,578 ordinary shares reserved for future issuance under our amended and restated 2014 Share Incentive Plan.
|
To the extent that any of these outstanding options are exercised or we issue additional shares under our equity incentive plans, there will be further
dilution to new investors. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that we raise
additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our shareholders.
S - 12
General
The following is a summary of the material Israeli tax laws applicable to us, and some Israeli Government
programs benefiting us. This section also contains a discussion of some Israeli tax consequences to persons owning our ordinary shares. This summary does not discuss all the aspects of Israeli tax law that may be relevant to a particular investor
in light of his or her personal investment circumstances or to some types of investors subject to special treatment under Israeli law. Examples of this kind of investor include traders in securities or persons that own, directly or indirectly, 10%
or more of our outstanding voting capital, all of whom are subject to special tax regimes not covered in this discussion. Some parts of this discussion are based on tax legislation which has not been subject to judicial or administrative
interpretation. The discussion should not be construed as legal or professional tax advice and does not cover all possible tax considerations.
SHAREHOLDERS ARE URGED TO CONSULT THEIR OWN TAX ADVISORS AS TO THE ISRAELI OR OTHER TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND
DISPOSITION OF OUR ORDINARY SHARES, INCLUDING, IN PARTICULAR, THE EFFECT OF ANY FOREIGN, STATE OR LOCAL TAXES.
General Corporate Tax Structure in Israel
Israeli resident companies are generally subject to corporate tax at the rate of 23% in 2022. However, the effective tax rate payable by
a company that derives income from a Benefited Enterprise or a Preferred Enterprise (as discussed below) may be considerably less. Capital gains derived by an Israeli resident company are subject to tax at the prevailing corporate tax rate.
Under Israeli tax legislation, a corporation will be considered as an “Israeli resident company” if it meets one of the following: (i)
it was incorporated in Israel; or (ii) the control and management of its business are exercised in Israel.
Law for the Encouragement of Industry (Taxes), 5729-1969
The Law for the Encouragement of Industry (Taxes), 5729-1969, generally referred to as the Industry Encouragement Law, provides several
tax benefits for “Industrial Companies.”
The Industry Encouragement Law defines an “Industrial Company” as a company resident in Israel and which was incorporated in Israel of
which 90% or more of its income in any tax year, other than income from defense loans, is derived from an “Industrial Enterprise” owned by it and which is located in Israel. An “Industrial Enterprise” is defined as an enterprise whose principal
activity in a given tax year is industrial production.
The following corporate tax benefits, among others, are available to Industrial Companies:
| • |
amortization over an eight-year period of the cost of purchased know-how and patents and rights to use a patent and know-how which are used for the development or advancement of the Industrial Enterprise;
|
| • |
under limited conditions, an election to file consolidated tax returns with related Israeli Industrial Companies; and
|
| • |
expenses related to a public offering are deductible in equal amounts over three years.
|
Although as of the date of this prospectus supplement, we do not have industrial production
activities, we may qualify as an Industrial Company in the future and may be eligible for the benefits described above.
S - 13
Tax Benefits and Grants for Research and Development
Israeli tax law allows, under certain conditions, a tax deduction for expenditures, including capital expenditures, for the year in
which they are incurred. Expenditures are deemed related to scientific research and development projects, if:
| • |
The expenditures are approved by the relevant Israeli government ministry, determined by the field of research;
|
| • |
The research and development must be for the promotion of the company; and
|
| • |
The research and development are carried out by or on behalf of the company seeking such tax deduction.
|
The amount of such deductible expenses is reduced by the sum of any funds received through government grants for the financing of such
scientific research and development projects. No deduction under these research and development deduction rules is allowed if such deduction is related to an expense invested in an asset depreciable under the general depreciation rules of the Israeli
Tax Ordinance, 1961. Expenditures not so approved are deductible in equal amounts over three years.
From time to time we may apply to the Israel Innovation Authority for approval to allow a tax deduction for all research and development
expenses during the year incurred. There can be no assurance that such application will be accepted.
Law for the Encouragement of Capital Investments, 5719-1959
The Law for the Encouragement of Capital Investments, 5719-1959, generally referred to as the Investment Law, provides certain
incentives for capital investments in production facilities (or other eligible assets) by “Industrial Enterprises” (as defined under the Investment Law).
Tax Benefits Prior to the 2005 Amendment
An investment program that is implemented in accordance with the provisions of the Investment Law prior to an amendment that became
effective in April 2005, or the 2005 Amendment, referred to as an “Approved Enterprise,” is entitled to certain benefits. A company that wished to receive benefits as an Approved Enterprise must have received approval from the Investment Center of
the Israeli Ministry of Economy and Industry, or the Investment Center. Each certificate of approval for an Approved Enterprise relates to a specific investment program in the Approved Enterprise, delineated both by the financial scope of the
investment and by the physical characteristics of the facility or the asset.
In general, an Approved Enterprise is entitled to receive a grant from the Government of Israel or an alternative package of tax
benefits, known as the alternative benefits track. The tax benefits from any certificate of approval relate only to taxable profits attributable to the specific Approved Enterprise. Income derived from activity that is not integral to the activity of
the Approved Enterprise does not enjoy tax benefits.
In addition, a company that has an Approved Enterprise program is eligible for further tax benefits if it qualifies as a Foreign
Investors’ Company, or FIC, which is a company with a level of foreign investment, as defined in the Investment Law, of more than 25%. The level of foreign investment is measured as the percentage of rights in the company (in terms of shares, rights
to profits, voting and appointment of directors), and of combined share and loan capital, that are owned, directly or indirectly, by persons who are not residents of Israel. The determination as to whether a company qualifies as an FIC is made on an
annual basis. We are currently not entitled to tax benefits for Approved Enterprise.
Tax Benefits Subsequent to the 2005 Amendment
The 2005 Amendment applies to new investment programs and investment programs commencing after 2004, but does not apply to investment
programs approved prior to April 1, 2005. The 2005 Amendment provides that terms and benefits included in any certificate of approval that was granted before the 2005 Amendment became effective (April 1, 2005) will remain subject to the provisions of
the Investment Law as in effect on the date of such approval. Pursuant to the 2005 Amendment, the Investment Center will continue to grant Approved Enterprise status to qualifying investments. The 2005 Amendment, however, limits the scope of
enterprises that may be approved by the Investment Center by setting criteria for the approval of a facility as an Approved Enterprise, such as provisions generally requiring that at least 25% of the Approved Enterprise’s income be derived from
exports.
S - 14
The 2005 Amendment provides that Approved Enterprise status will only be necessary for receiving cash grants. As a result, it was no
longer necessary for a company to obtain Approved Enterprise status in order to receive the tax benefits previously available under the alternative benefits track. Rather, a company may claim the tax benefits offered by the Investment Law directly in
its tax returns, provided that its facilities meet the criteria for tax benefits set forth in the amendment. Companies are entitled to approach the Israel Tax Authority for a pre-ruling regarding their eligibility for benefits under the Investment
Law, as amended.
In order to receive the tax benefits, the 2005 Amendment states that a company must make an investment which meets all of the
conditions, including exceeding a minimum investment amount specified in the Investment Law. Such investment allows a company to receive “Benefited Enterprise” status, and may be made over a period of no more than three years from the end of the year
in which the company requested to have the tax benefits apply to its Benefited Enterprise. Where the company requests to apply the tax benefits to an expansion of existing facilities, only the expansion will be considered to be a Benefited Enterprise
and the company’s effective tax rate will be the weighted average of the applicable rates. In this case, the minimum investment required in order to qualify as a Benefited Enterprise is required to exceed a certain percentage of the value of the
company’s production assets before the expansion.
The extent of the tax benefits available under the 2005 Amendment to qualifying income of a Benefited Enterprise depend on, among other
things, the geographic location in Israel of the Benefited Enterprise. The location will also determine the period for which tax benefits are available. Such tax benefits include an exemption from corporate tax on undistributed income for a period of
between two to 10 years, depending on the geographic location of the Benefited Enterprise in Israel, and a reduced corporate tax rate of between 10% and the applicable corporate tax for the remainder of the benefits period, depending on the level of
foreign investment in the company in each year. A company qualifying for tax benefits under the 2005 Amendment which pays a dividend out of income derived by its Benefited Enterprise during the tax exemption period will be subject to corporate tax in
respect of the gross amount of the dividend at the otherwise applicable corporate tax rate or a lower rate in the case of a qualified FIC which is at least 49% owned by non-Israeli residents. Dividends paid out of income attributed to a Benefited
Enterprise are generally subject to withholding tax at source at the rate of 20% or such lower rate as may be provided in an applicable tax treaty.
The benefits available to a Benefited Enterprise are subject to the fulfillment of conditions stipulated in the Investment Law and its regulations. If a
company does not meet these conditions, it may be required to refund the amount of tax benefits, as adjusted by the Israeli consumer price index, and interest, or other monetary penalties.
We applied for tax benefits as a “Benefited Enterprise” with 2012 as a “Year of Election.” We may be entitled to tax benefits under this
regime once we are profitable for tax purposes and subject to the fulfillment of all the relevant conditions. If we do not meet these conditions, the tax benefits may not be applicable which would result in adverse tax consequences to us.
Alternatively, and subject to the fulfillment of all the relevant conditions, we may elect in the future to irrevocably waive the tax benefits available for Benefited Enterprise and claim the tax benefits available to Preferred Enterprise under the
2011 Amendment (as detailed below).
Tax Benefits Under the 2011 Amendment
The Investment Law was significantly amended as of January 1, 2011, or the 2011 Amendment. The 2011 Amendment introduced new benefits to
replace those granted in accordance with the provisions of the Investment Law in effect prior to the 2011 Amendment.
The 2011 Amendment introduced new tax benefits for income generated by a “Preferred Company” through its “Preferred Enterprise,” in
accordance with the definition of such term in the Investment Law, which generally means that a “Preferred Company” is an industrial company meeting certain conditions (including a minimum threshold of 25% export).
S - 15
A Preferred Company is entitled to a reduced flat tax rate with respect to the income attributed to the Preferred Enterprise, at the
following rates:
|
Tax Year
|
|
|
Development Region “A”
|
|
|
Other
Areas
within
Israel
|
|
||
|
2011 – 2012
|
|
|
|
10
|
%
|
|
|
15
|
%
|
|
2013
|
|
|
|
7
|
%
|
|
|
12.5
|
%
|
|
2014 – 2016
|
|
|
|
9
|
%
|
|
|
16
|
%
|
|
2017 and thereafter
|
|
|
|
7.5
|
%
|
|
|
16
|
%
|
Dividends distributed from income which is attributed to a “Preferred Enterprise” will be subject to withholding
tax at source at the following rates: (i) Israeli resident corporations — 0%, (ii) Israeli resident individuals — 20% in 2022 (iii) non-Israeli residents — may be reduced to 4% in 2022, subject to certain conditions under the Investment Law and to
a reduced tax rate under the provisions of an applicable double tax treaty.
Under the 2011 Amendment, a company located in Development Region “A” may be entitled to cash grants and the provision of loans under
certain conditions, if approved. The rates for grants and loans shall not be fixed, but up to 20% of the amount of the approved investment (may be increased by an additional 4%). In addition, a company owning a Preferred Enterprise under the Grant
Track may be entitled also to the tax benefits which are prescribed for a Preferred Company.
The termination or substantial reduction of any of the benefits available under the Investment Law could materially increase our tax
liabilities.
We are currently not entitled to tax benefits for a Preferred Enterprise.
Taxation of Our Shareholders
Capital Gains
Capital gain tax is imposed on the disposition of capital assets by an Israeli resident, and on the disposition of such assets by a
non-Israeli resident if those assets are either (i) located in Israel; (ii) are shares or a right to a share in an Israeli resident corporation, or (iii) represent, directly or indirectly, rights to assets located in Israel. The Israeli Tax Ordinance
distinguishes between “Real Gain” and the “Inflationary Surplus.” Real Gain is the excess of the total capital gain over Inflationary Surplus computed generally on the basis of the increase in the Israeli consumer price index between the date of
purchase and the date of disposition. Inflationary Surplus is not currently subject to tax in Israel.
Real Gain accrued by individuals on the sale of our ordinary shares will be taxed at the rate of 25%. However, if the individual
shareholder is a “Controlling Shareholder” (i.e., a person who holds, directly or indirectly, alone or together with another, 10% or more of one of the Israeli resident company’s means of control) at the time of sale or at any time during the
preceding 12-month period, such gain will be taxed at the rate of 30%.
Real Gain derived by corporations will be generally subject to the corporate tax rate of 23% in 2022.
Individual and corporate shareholder dealing in securities in Israel are taxed at the tax rates applicable to
business income —23% for corporations in 2022, and a marginal tax rate of up to 50% for individuals, including an excess tax.
S - 16
Notwithstanding the foregoing, capital gain derived from the sale of our ordinary shares by a non-Israeli shareholder may be exempt
under the Israeli Tax Ordinance from Israeli capital gain tax provided that the seller does not have a permanent establishment in Israel to which the derived capital gain is attributed. However, non-Israeli corporations will not be entitled to the
foregoing exemption if more than 25% of its means of control are held, directly and indirectly, by Israeli residents, and Israeli residents are entitled to 25% or more of the revenues or profits of the corporation, directly or indirectly. In
addition, such exemption would not be available to a person whose gains from selling or otherwise disposing of the securities are deemed to be business income.
In addition, the sale of shares may be exempt from Israeli capital gain tax under the provisions of an applicable tax treaty. For
example, the U.S.-Israel Double Tax Treaty exempts U.S. residents from Israeli capital gain tax in connection with such sale, provided (i) the U.S. resident owned, directly or indirectly, less than 10% of an Israeli resident company’s voting power at
any time within the 12-month period preceding such sale; (ii) the seller, being an individual, is present in Israel for a period or periods of less than 183 days during the taxable year; and (iii) the capital gain from the sale was not derived
through a permanent establishment of the U.S. resident in Israel.
In some instances where our shareholders may be liable for Israeli tax on the sale of their ordinary shares, the payment of the
consideration may be subject to the withholding of Israeli tax at source at a rate of 25% if the seller is an individual and at the corporate tax rate (23% in 2022) if the seller is a corporation. Shareholders may be required to demonstrate that they
are exempt from tax on their capital gains in order to avoid withholding at source at the time of sale.
At the sale of securities traded on a stock exchange a detailed return, including a computation of the tax due, must be filed and an
advanced payment must be paid on January 31 and July 31 of every tax year in respect of sales of securities made within the previous six months. However, if all tax due was withheld at source according to applicable provisions of the Israeli Tax
Ordinance and regulations promulgated thereunder, the aforementioned return need not be filed and no advance payment must be paid. Capital gain is also reportable on the annual income tax return.
Dividends
We have never paid cash dividends. A distribution of a dividend by our company from income attributed to a Benefited Enterprise will
generally be subject to withholding tax in Israel at a rate of 20% unless a reduced tax rate is provided under an applicable tax treaty. A distribution of a dividend by our company from income attributed to a Preferred Enterprise will generally be
subject to withholding tax in Israel at the following tax rates: Israeli resident individuals — 20%; Israeli resident companies — 0% for a Preferred Enterprise; Non-Israeli residents — 20%, subject to a reduced rate under the provisions of any
applicable double tax treaty. A distribution of dividends from income, which is not attributed to a Preferred Enterprise to an Israeli resident individual, will generally be subject to withholding tax at a rate of 25%, or 30% if the dividend
recipient is a “Controlling Shareholder” (as defined above) at the time of distribution or at any time during the preceding 12-month period. If the recipient of the dividend is an Israeli resident corporation, such dividend will not be subject to
Israeli tax provided the income from which such dividend is distributed was derived or accrued within Israel.
The Israeli Tax Ordinance provides that a non-Israeli resident (either individual or corporation) is generally subject to Israeli
withholding tax on the receipt of dividends at the rate of 25% (30% if the dividends recipient is a “Controlling Shareholder” (as defined above), at the time of distribution or at any time during the preceding 12-month period); those rates may be
subject to a reduced rate under the provisions of an applicable double tax treaty. Under the U.S.-Israel Double Tax Treaty, the following withholding rates will apply in respect of dividends distributed by an Israeli resident company to a U.S.
resident: (i) if the U.S. resident is a corporation which holds during that portion of the taxable year which precedes the date of payment of the dividend and during the whole of its prior taxable year (if any), at least 10% of the outstanding shares
of the voting share capital of the Israeli resident paying corporation and not more than 25% of the gross income of the Israeli resident paying corporation for such prior taxable year (if any) consists of certain type of interest or dividends — the
rate is 12.5%, (ii) if both the conditions mentioned in clause (i) above are met and the dividend is paid from an Israeli resident company’s income which was entitled to a reduced tax rate applicable to an Approved Enterprise — the rate is 15% and
(iii) in all other cases, the rate is 25%. The aforementioned rates under the U.S.-Israel Double Tax Treaty will not apply if the dividend income was derived through a permanent establishment of the U.S. resident in Israel.
S - 17
A non-Israeli resident who receives dividends from which tax was withheld is generally exempt from the obligation to file tax returns in
Israel with respect to such income, provided that (i) such income was not generated from a business conducted in Israel by the taxpayer, and (ii) the taxpayer has no other taxable sources of income in Israel with respect to which a tax return is
required to be filed.
Dividends are generally subject to Israeli withholding tax at a rate of 25% so long as the shares are registered with a nominee company
(whether or not the recipient is a “Controlling Shareholder,” as defined above), unless relief is provided in a treaty between Israel and the shareholder’s country of residence and provided that a certificate from the Israel Tax Authority allowing
for a reduced withholding tax rate is obtained in advance.
Excess Tax
Individuals who are subject to tax in Israel are also subject to an additional tax at a rate of 3% on annual income exceeding NIS
663,240 for 2022, linked to the annual change in the Israeli consumer price index, including, but not limited to income derived from, dividends, interest and capital gains.
Foreign Exchange Regulations
Non-residents of Israel who hold our ordinary shares are able to receive any dividends, and any amounts payable upon the dissolution,
liquidation and winding up of our affairs, repayable in non-Israeli currency at the rate of exchange prevailing at the time of conversion. However, Israeli income tax is generally required to have been paid or withheld on these amounts. In addition,
the statutory framework for the potential imposition of currency exchange control has not been eliminated and may be restored at any time by administrative action.
Estate and Gift Tax
Israeli law presently does not impose estate or gift taxes.
U.S. Federal Income Tax Considerations
The following discussion describes certain material U.S. federal income tax consequences to
U.S. Holders (as defined below) under present law of an investment in our ordinary shares. This discussion applies only to U.S. Holders that hold our ordinary shares as capital assets within the meaning of Section 1221 of the Internal Revenue Code of
1986, as amended, or the Code, and that have the U.S. dollar as their functional currency.
This discussion is based on the tax laws of the United States, including the Code, as in
effect on the date hereof and on U.S. Treasury regulations as in effect or, in some cases, as proposed, on the date hereof, as well as judicial and administrative interpretations thereof available on or before such date. All of the foregoing
authorities are subject to change, which change could apply retroactively and could affect the tax consequences described below. This summary does not address any estate or gift tax consequences, the alternative minimum tax, the Medicare tax on net
investment income or any state, local, or non-U.S. tax consequences. The following discussion neither deals with the tax consequences to any particular investor nor describes all of the tax consequences applicable to persons in special tax situations
such as:
| • |
banks;
|
| • |
certain financial institutions;
|
| • |
insurance companies;
|
| • |
regulated investment companies;
|
S - 18
| • |
real estate investment trusts;
|
| • |
broker-dealers;
|
| • |
traders that elect to mark to market;
|
| • |
U.S. expatriates;
|
| • |
tax-exempt entities;
|
| • |
persons holding our ordinary shares as part of a straddle, hedging, constructive sale, conversion or integrated transaction;
|
| • |
persons that actually or constructively (including through the ownership of our warrants) own 10% or more of our share capital (by vote or value);
|
| • |
persons that are resident or ordinarily resident in or have a permanent establishment in a jurisdiction outside the United States;
|
| • |
persons who acquired our ordinary shares pursuant to the exercise of any employee share option or otherwise as compensation;
|
| • |
persons subject to special tax accounting rules as a result of any item of gross income with respect to our ordinary shares being taken into account in an applicable financial statement; or
|
| • |
pass-through entities, or persons holding our ordinary shares through pass-through entities.
|
INVESTORS ARE URGED TO CONSULT THEIR TAX ADVISORS ABOUT THE APPLICATION OF THE U.S. FEDERAL
TAX RULES TO THEIR PARTICULAR CIRCUMSTANCES AS WELL AS THE STATE, LOCAL, NON-U.S. AND OTHER TAX CONSEQUENCES TO THEM OF AN INVESTMENT IN OUR ORDINARY SHARES.
The discussion below of the U.S. federal income tax consequences to “U.S. Holders” will apply
to you if you are the beneficial owner of our ordinary shares and you are, for U.S. federal income tax purposes,
| • |
an individual who is a citizen or resident of the United States;
|
| • |
a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes) created or organized in the United States or under the laws of the United States, any state thereof or the District of Columbia;
|
| • |
an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or
|
| • |
a trust that (i) is subject to the primary supervision of a court within the United States and the control of one or more U.S. persons for all substantial decisions or (ii) has a valid election in effect under applicable U.S. Treasury
regulations to be treated as a U.S. person.
|
If an entity or other arrangement treated as a partnership for U.S. federal income tax
purposes holds our ordinary shares, the tax treatment of a partner will generally depend upon the status of the partner and the activities of the partnership. A person that would be a U.S. Holder if it held our ordinary shares directly and that is a
partner of a partnership holding our ordinary shares is urged to consult its own tax advisor.
Passive Foreign Investment Company
A non-U.S. entity treated as a corporation for U.S. federal income tax purposes will
generally be a passive foreign investment company (“PFIC”) for U.S. federal income tax purposes for any taxable year if either:
| • |
at least 75% of its gross income for such year is passive income (such as interest income); or
|
| • |
at least 50% of the value of its assets (based on an average of the quarterly values of the assets) during such year is attributable to assets that produce passive income or are held for the production of passive income.
|
S - 19
For this purpose, we will be treated as owning our proportionate share of the assets and
earning our proportionate share of the income of any other entity treated as a corporation for U.S. federal income tax purposes in which we own, directly or indirectly, 25% or more (by value) of the stock.
For our 2019 through 2021 taxable years, we generated revenue under our then collaboration
agreement with Perrigo UK Finco Limited Partnership, or Perrigo, for the development of a generic product candidate. In 2021 we sold our rights to this and other generic products and will unconditionally receive further revenue over 24 months in lieu
of our share in the collaboration agreements with respect to these products. Starting in 2021, we began generating revenue under our license agreements with Galderma for Twyneo®, and Epsolay®. See “Item 4. Information on the Company – B. Business
Overview”.. Though the application of the relevant rules governing the characterization of the foregoing revenue for purposes of the PFIC income test is uncertain, we intend to take the position that, based on our involvement and management
contributions throughout the development process, such revenue is non-passive for PFIC purposes. As a result, assuming we continue to earn substantial revenue from such agreements as anticipated and based on the current and anticipated value and
composition of our income and assets, we do not expect that we will be treated as a PFIC for U.S. federal income tax purposes for our current taxable year or for foreseeable future years. However, there are substantial factual and legal ambiguities
regarding the nature of the revenue and the application of the relevant PFIC rules, and thus, the determination that such revenue is non-passive is not without doubt, and alternative characterizations are possible.
A separate determination must be made after the close of each taxable year as to whether we
were a PFIC for that year. Because the value of our assets for purposes of the PFIC test will generally be determined by reference to the market price of our ordinary shares, our PFIC status may depend in part on the market price of our ordinary
shares, which may fluctuate significantly. In addition, there may be certain other ambiguities in applying the PFIC test to us. No rulings from the U.S. Internal Revenue Service, or the IRS, however, have been or will be sought with respect to our
status as a PFIC. If the IRS were to assert that, contrary to our expectation, we are a PFIC in the current taxable year or a future year, there would be adverse tax consequences to investors, including those described below. Potential investors are
strongly advised to consult their own advisors regarding the consequences to them if we were to be considered a PFIC.
If we are a PFIC for any taxable year during your holding period for our ordinary shares, we
generally will continue to be treated as a PFIC with respect to your investment in our ordinary shares for all succeeding years during which you hold our ordinary shares. Certain elections (such as a deemed sale election) may be available under
certain circumstances.
For each taxable year that we are treated as a PFIC with respect to you, you will be subject
to special tax rules with respect to any “excess distribution” (as defined below) you receive and any gain you realize from a sale or other disposition (including a pledge) of our ordinary shares, unless you make a valid “mark-to-market” election as
discussed below. Distributions you receive in a taxable year that are greater than 125% of the average annual distributions you received during the shorter of the three preceding taxable years or your holding period for our ordinary shares will be
treated as an excess distribution. Under these special tax rules:
| • |
the excess distribution or gain will be allocated ratably over your holding period for our ordinary shares;
|
| • |
the amount allocated to the current taxable year, and any taxable years in your holding period prior to the first taxable year in which we were a PFIC, will be treated as ordinary income; and
|
| • |
the amount allocated to each other taxable year will be subject to the highest tax rate in effect for individuals or corporations, as applicable, for each such year and the interest charge generally applicable to underpayments of tax will
be imposed on the resulting tax attributable to each such year.
|
The tax liability for amounts allocated to taxable years prior to the year of disposition
or excess distribution cannot be offset by any net operating losses, and gains (but not losses) realized on the sale of our ordinary shares cannot be treated as capital gains, even if you hold our ordinary shares as capital assets.
If we are treated as a PFIC with respect to you for any taxable year, to the extent any of
our subsidiaries are also PFICs, you may be deemed to own shares in such lower-tier PFICs that are directly or indirectly owned by us in that proportion which the value of our ordinary shares you own bears to the value of all of our ordinary shares,
and you may be subject to the adverse tax consequences described above with respect to the shares of such lower-tier PFICs you would be deemed to own. As a result, you may incur liability for any excess distribution described above if we receive a
distribution from our lower-tier PFICs or if any shares in such lower-tier PFICs are disposed of (or deemed disposed of). You should consult your tax advisor regarding the application of the PFIC rules to any of our subsidiaries.
S - 20
A U.S. Holder of “marketable stock” (as defined below) in a PFIC may make a mark-to-market
election for such stock to elect out of the tax treatment discussed above. If you make a valid mark-to-market election for our ordinary shares, you will include in income for each year that we are treated as a PFIC with respect to you an amount equal
to the excess, if any, of the fair market value of our ordinary shares as of the close of your taxable year over your adjusted basis in such ordinary shares. You will be allowed a deduction for the excess, if any, of the adjusted basis of our
ordinary shares over their fair market value as of the close of the taxable year. However, deductions will be allowable only to the extent of any net mark-to-market gains on our ordinary shares included in your income for prior taxable years. Amounts
included in your income under a mark-to-market election, as well as gain on the actual sale or other disposition of our ordinary shares, will be treated as ordinary income. Ordinary loss treatment will also apply to the deductible portion of any
mark-to-market loss on our ordinary shares, as well as to any loss realized on the actual sale or disposition of our ordinary shares, to the extent the amount of such loss does not exceed the net mark-to-market gains for such ordinary shares
previously included in income. Your basis in our ordinary shares will be adjusted to reflect any such income or loss amounts. If you make a mark-to-market election, any distributions we make would generally be subject to the rules discussed below
under “— Taxation of Dividends and Other Distributions on our Ordinary Shares,” except the lower rates applicable to qualified dividend income would not apply.
The mark-to-market election is available only for “marketable stock,” which is stock that
is regularly traded on a qualified exchange or other market, as defined in applicable U.S. Treasury regulations. Our ordinary shares are listed on the Nasdaq Global Market. Because a mark-to-market election cannot be made for equity interests in any
lower-tier PFICs we own, you generally will continue to be subject to the PFIC rules with respect to your indirect interest in any investments held by us that are treated as an equity interest in a PFIC for U.S. federal income tax purposes. The
Nasdaq Global Market is a qualified exchange, but there can be no assurance that the trading in our ordinary shares will be sufficiently regular to qualify our ordinary shares as marketable stock. You should consult your tax advisor as to the
availability and desirability of a mark-to-market election, as well as the impact of such election on interests in any lower-tier PFICs.
Alternatively, if a non-U.S. entity treated as a corporation is a PFIC, a holder of shares
in that entity may avoid taxation under the PFIC rules described above regarding excess distributions and recognized gains by making a “qualified electing fund” election to include in income its share of the entity’s income on a current basis.
However, you may make a qualified electing fund election with respect to your ordinary shares only if we furnish you annually with certain tax information, and we currently do not intend to prepare or provide such information.
A U.S. Holder of a PFIC may be required to file an IRS Form 8621. If we are a PFIC, you
should consult your tax advisor regarding any reporting requirements that may apply to you. You are urged to consult your tax advisor regarding the application of the PFIC rules to an investment in ordinary shares.
YOU ARE STRONGLY URGED TO CONSULT YOUR TAX ADVISOR REGARDING THE IMPACT ON YOUR INVESTMENT
IN OUR ORDINARY SHARES IF WE WERE TO BE CONSIDERED A PFIC AS WELL AS THE APPLICATION OF THE PFIC RULES AND THE POSSIBILITY OF MAKING A MARK-TO-MARKET ELECTION.
Taxation of Dividends and Other Distributions on our Ordinary Shares
Subject to the PFIC rules discussed above, the gross amount of any distributions we make to
you (including the amount of any tax withheld) with respect to our ordinary shares generally will be includible in your gross income as dividend income on the date of receipt by the holder, but only to the extent the distribution is paid out of our
current or accumulated earnings and profits (as determined under U.S. federal income tax principles). The dividends will not be eligible for the dividends-received deduction allowed to corporations in respect of dividends received from other U.S.
corporations. To the extent the amount of the distribution exceeds our current and accumulated earnings and profits (as determined under U.S. federal income tax principles), such excess amount will be treated first as a tax-free return of your tax
basis in your ordinary shares, and then, to the extent such excess amount exceeds your tax basis in your ordinary shares, as capital gain. We currently do not, and we do not intend to, calculate our earnings and profits under U.S. federal income tax
principles. Therefore, you should expect that a distribution will generally be reported as a dividend even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above.
S - 21
Certain non-corporate U.S. Holders, including individual U.S. Holders, dividends may be taxed
at the lower capital gain rates applicable to “qualified dividend income.” Distributions, if any, on our ordinary shares received by such U.S. Holders generally will be qualified
dividend income if: (i) either our ordinary shares are readily tradable on an established securities market in the United States (such as the Nasdaq Global Market), or we are eligible for the benefits of the Treaty (as defined below) (ii) we are
neither a PFIC nor treated as such with respect to you (as discussed above) for either the taxable year in which the dividend was paid or the preceding taxable year, (iii) certain holding period requirements are met and (iv) you are not under an
obligation to make related payments with respect to positions in substantially similar or related property.
The amount of any distribution paid in a currency other than U.S. dollars will be equal to the
U.S. dollar value of such currency on the date such distribution is includible in your income, regardless of whether the payment is in fact converted into U.S. dollars at that time. The amount of any distribution of property other than cash will be
the fair market value of such property on the date of distribution.
Any dividends will constitute foreign source income for foreign tax credit limitation
purposes. If the dividends are taxed as qualified dividend income (as discussed above), the amount of the dividend taken into account for purposes of calculating the foreign tax credit limitation will in general be limited to the gross amount of the
dividend, multiplied by the reduced tax rate applicable to qualified dividend income and divided by the highest tax rate normally applicable to dividends. The limitation on foreign taxes eligible for credit is calculated separately with respect to
specific classes of income. For this purpose, dividends distributed by us with respect to our ordinary shares will generally constitute “passive category income.”
If Israeli withholding taxes apply to any dividends paid to you with respect to our ordinary
shares, subject to certain conditions and limitations, such withholding taxes may be treated as foreign taxes eligible for credit against your U.S. federal income tax liability. Instead of claiming a credit, you may elect to deduct such taxes in
computing taxable income, subject to applicable limitations. If a refund of the tax withheld is available under the applicable laws of Israel or under the Israel-U.S. income tax treaty, or the Treaty, the amount of tax withheld that is refundable
will not be eligible for such credit against your U.S. federal income tax liability (and will not be eligible for the deduction against your U.S. federal taxable income). The rules relating to the determination of the foreign tax credit are complex,
and you should consult your tax advisor regarding the availability of a foreign tax credit in your particular circumstances, including the effects of the Treaty.
Taxation of Disposition of our Ordinary Shares
Subject to the PFIC rules discussed above, upon a sale or other disposition of our ordinary
shares, you will generally recognize capital gain or loss for U.S. federal income tax purposes in an amount equal to the difference between the amount realized (including the amount of any tax withheld) and your tax basis in such ordinary shares. If
the consideration you receive for our ordinary shares is not paid in U.S. dollars, the amount realized will be the U.S. dollar value of the payment received determined by reference to the spot rate of exchange on the date of the sale or other
disposition. However, if our ordinary shares are treated as traded on an “established securities market” and you are either a cash basis taxpayer or an accrual basis taxpayer that has made a special election (which must be applied consistently from
year to year and cannot be changed without the consent of the IRS), you will determine the U.S. dollar value of the amount realized in a non-U.S. dollar currency by translating the amount received at the spot rate of exchange on the settlement date
of the sale. If you are an accrual basis taxpayer that is not eligible to or does not elect to determine the amount realized using the spot rate on the settlement date, you will recognize foreign currency gain or loss to the extent of any difference
between the U.S. dollar amount realized on the date of sale or disposition and the U.S. dollar value of the currency received at the spot rate on the settlement date.
Your tax basis in our ordinary shares generally will equal the cost of such ordinary shares.
Any gain or loss on the sale or other disposition of our ordinary shares will generally be treated as U.S. source income or loss and treated as long-term capital gain or loss if your holding period in our ordinary shares at the time of the
disposition exceeds one year. Accordingly, in the event any Israeli tax (including withholding tax) is imposed upon the sale or other disposition, you may not be able to utilize foreign tax credit unless you have foreign source income or gain in the
same category from other sources. Long-term capital gain of non-corporate U.S. Holders generally will be subject to U.S. federal income tax at reduced tax rates. The deductibility of capital losses is subject to significant limitations.
S - 22
Information Reporting and Backup Withholding
Dividend payments with respect to our ordinary shares or warrants and proceeds from the
sale, exchange or redemption of our ordinary shares or warrants may be subject to information reporting to the IRS and possible U.S. backup withholding. Backup withholding will not apply, however, to a U.S. Holder that furnishes a correct taxpayer
identification number and makes any other required certification or that is otherwise exempt from backup withholding. U.S. Holders that are required to establish their exempt status generally must provide such certification on IRS Form W-9. You
should consult your tax advisor regarding the application of the U.S. information reporting and backup withholding rules.
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be
credited against your U.S. federal income tax liability, and you may obtain a refund of any excess amounts withheld under the backup withholding rules by filing the appropriate claim for refund with the IRS and furnishing any required information in
a timely manner.
Information with respect to Foreign Financial Assets
Certain U.S. Holders may be required to report information relating to an interest in our
ordinary shares, subject to certain exceptions (including an exception for ordinary shares held in accounts maintained by certain financial institutions). Penalties can apply if U.S. Holders fail to satisfy such reporting requirements. You should
consult your tax advisor regarding the effect, if any, of this requirement on your ownership and disposition of our ordinary shares.
THE SUMMARY OF U.S. FEDERAL INCOME TAX CONSEQUENCES SET OUT ABOVE IS FOR GENERAL
INFORMATIONAL PURPOSES ONLY. INVESTORS ARE URGED TO CONSULT THEIR TAX ADVISORS ABOUT THE APPLICATION OF THE U.S. FEDERAL TAX RULES TO THEIR PARTICULAR CIRCUMSTANCES AS WELL AS THE STATE, LOCAL, NON-U.S. AND OTHER TAX CONSEQUENCES TO THEM OF AN
INVESTMENT IN OUR ORDINARY SHARES.
S - 23
PLAN OF DISTRIBUTION
We have entered into an Open Market Sale AgreementSM, or the Sale Agreement,
with Jefferies under which we may offer and sell our ordinary shares from time to time through Jefferies acting as agent. Sales of our ordinary shares, if any, under this prospectus supplement and the accompanying prospectus will be made by any
method that is deemed to be an “at the market offering” as defined in Rule 415(a)(4) promulgated under the Securities Act, including sales made directly on or through the Nasdaq Global Market or any other existing trading market for our ordinary
shares.
Each time we wish to issue and sell our ordinary shares under the Sale Agreement, we will notify Jefferies of the number of shares to be issued, the dates on which such sales are anticipated to be made,
any limitation on the number of shares to be sold in any one day and any minimum price below which sales may not be made. Once we have so instructed Jefferies, unless Jefferies declines to accept the terms of such notice, Jefferies has agreed to
use its commercially reasonable efforts consistent with its normal trading and sales practices to sell such shares up to the amount specified on such terms. The obligations of Jefferies under the sales agreement to sell our ordinary shares are
subject to a number of conditions that we must meet.
The settlement of sales of shares between us and Jefferies is generally anticipated to occur on the second trading day following the date on which the sale was made. Sales of our ordinary shares as
contemplated in this prospectus supplement will be settled through the facilities of The Depository Trust Company or by such other means as we and Jefferies may agree upon. There is no arrangement for funds to be received in an escrow, trust or
similar arrangement.
We will pay Jefferies a commission of 3.0% of the gross proceeds of any shares sold through it pursuant to this prospectus supplement and the Sale Agreement
and reimburse Jefferies for up to $75,000 of its expenses in connection with the execution of the Sale Agreement and, upon the occurrence of certain events specified in the Sale Agreement, up to $15,000 in connection with each such specified event,
including, in each case, fees and disbursements to its legal counsel. In accordance with Financial Industry Regulatory Authority, Inc. Rule 5110, these reimbursed fees and expenses are deemed sales compensation to Jefferies in connection with this
offering. The estimated offering expenses payable by us, in addition to such commission and reimbursed expenses, are approximately $75,000, which includes legal, accounting and printing costs and various other fees associated with registering the
ordinary shares. The remaining sales proceeds, after deducting any other transaction fees, will equal our net proceeds from the sale of such shares. Because there is no minimum offering amount required as a condition to close this offering, the
actual total public offering amount, commissions and proceeds to us, if any, are not determinable at this time.
Jefferies will provide written confirmation to us before the open of trading on the Nasdaq Global Market on the day following each day on which ordinary
shares are sold by Jefferies for us under the Sale Agreement. Each confirmation will include the number of shares sold on that day, the aggregate gross proceeds of such sales and the net proceeds to us.
In connection with the sale of our ordinary shares on our behalf, Jefferies may be deemed to be an “underwriter” within the meaning of the Securities Act and
the compensation of Jefferies will be deemed to be underwriting commissions or discounts. We have agreed in the Sale Agreement to indemnify Jefferies against certain liabilities, including liabilities under the Securities Act. We have also agreed to
contribute to payments Jefferies may be required to make in respect of such liabilities.
The offering of ordinary shares pursuant to the Sale Agreement will terminate upon the earlier of (i) the sale of all of the ordinary shares subject to the Sale
Agreement and (ii) the termination of the Sale Agreement according to its terms by either Jefferies or us. We and Jefferies may each terminate the Sale Agreement at any time upon ten days’ prior notice.
This summary of the material provisions of the Sale Agreement does not purport to be a complete statement of its terms and conditions. A copy of the Sale Agreement is
filed as an exhibit to a report on Form 6-K filed under the Exchange Act and incorporated by reference in this prospectus supplement.
Jefferies and its affiliates have from time to time provided, and may in the future provide, various investment banking, commercial banking, financial
advisory and other services to us and our affiliates and may in the future receive customary fees. In the course of its business, Jefferies may actively trade our securities for its own account or for the accounts of customers, and, accordingly,
Jefferies may at any time hold long or short positions in such securities.
This prospectus supplement and the accompanying prospectus in electronic format may be made available on a website maintained by Jefferies and Jefferies may
distribute this prospectus supplement and the accompanying prospectus electronically.
This document does not constitute a prospectus under the Israeli Securities Law, 5728-1968, or the Securities Law, and has not been filed with or approved by
the Israel Securities Authority.
S - 24
Certain legal matters with respect to Israeli law and with respect to the validity of the ordinary shares under Israeli law will be passed upon
for us by Gross & Co. Certain legal matters with respect to U.S. law will be passed upon for us by Latham & Watkins LLP, New York, New York. Covington & Burling LLP, New York, New York, is acting as counsel for the sales agent in
connection with this offering with respect to U.S. law, and Gornitzky & Co., Tel Aviv, Israel, is acting as counsel for the sales agent in connection with this offering with respect to Israeli law.
The financial statements incorporated in this prospectus by reference to our Annual Report on Form 20-F for the year ended December 31, 2021 have been so incorporated in reliance
on the report of Kesselman & Kesselman, Certified Public Accountants (Isr.), a member firm of PricewaterhouseCoopers International Limited, an independent registered public accounting firm, given on the authority of said firm as experts in
auditing and accounting.
We have filed with the SEC a registration statement on Form F-3 under the Securities Act with respect to the securities offered by this prospectus supplement. However, as is
permitted by the rules and regulations of the SEC, this prospectus supplement and the accompanying prospectus, which are part of our registration statement on Form F-3, omit certain non-material information, exhibits, schedules and undertakings set
forth in the registration statement. For further information about us, and the securities offered by this prospectus, please refer to the registration statement.
We are subject to the reporting requirements of the Exchange Act that are applicable to a foreign private issuer. In accordance with the Exchange Act, we file reports, including
annual reports on Form 20-F containing financial statements audited by an independent accounting firm. We also furnish to the SEC, under cover of Reports of Foreign Private Issuer on Form 6-K, material information required to be made public by us
or filed by us with and made public by any stock exchange or distributed by us to our shareholders.
The SEC maintains an Internet site that contains reports, information statements and other information regarding issuers, such as us, that file electronically with the SEC
(http://www.sec.gov).
As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements to shareholders and our officers,
directors and principal shareholders are exempt from the “short-swing profits” reporting and liability provisions contained in Section 16 of the Exchange Act and related Exchange Act rules. In addition, we are not required under the Exchange Act to
file periodic reports and financial statements as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act.
We file annual and special reports and other information with the SEC (File Number 001-38367). These filings contain important
information which does not appear in this prospectus. The SEC allows us to “incorporate by reference” information into this prospectus, which means that we can disclose important information to you by referring you to other documents which we have
filed or will file with the SEC. We are incorporating by reference in this prospectus the documents listed below and all amendments or supplements we may file to such documents, as well as any future filings we may make with the SEC on Form 20-F
under the Exchange Act before the time that all of the securities offered by this prospectus have been sold or de-registered:
| • |
our Annual Report on Form 20-F for the fiscal year ended on
December 31, 2021, filed with the SEC on April 4, 2022, as amended by Amendment No. 1 to Form 20-F, filed with the SEC on April 7, 2022;
and
|
| • |
the description of our ordinary shares contained under the heading “Item 1. Description of Registrant’s Securities to be Registered” in our registration statement on Form 8-A, as filed with the SEC on January 26, 2018, including any subsequent amendment or any report filed for the purpose of updating such
description.
|
S - 25
In addition, all subsequent annual reports on Form 20-F filed after the effective date of this registration statement and prior to the
termination of this offering and any reports on Form 6-K subsequently submitted to the SEC or portions thereof that we specifically identify in such forms as being incorporated by reference into the registration statement of which this prospectus
forms a part, shall be considered to be incorporated into this prospectus by reference and shall be considered a part of this prospectus from the date of filing or submission of such documents.
Certain statements in and portions of this prospectus supplement update and replace information in the above listed documents
incorporated by reference. Likewise, statements in or portions of a future document incorporated by reference in this prospectus supplement may update and replace statements in and portions of this prospectus supplement or the above listed
documents.
We will provide you without charge, upon your written or oral request, a copy of any of the documents incorporated by reference in this
prospectus, other than exhibits to such documents which are not specifically incorporated by reference into such documents. Please direct your written or telephone requests to Sol-Gel Technologies Ltd., 7 Golda Meir Street, Weizmann Science Park,
Ness Ziona, 7403650, Israel, Attn: Gilad Mamlok, telephone number +972 (8) 931-3433. You may also obtain information about us by visiting our website at www.sol-gel.com. Information contained in our website is not part of this prospectus. We have
included our website address in this prospectus solely as an inactive textual reference.
We are incorporated under the laws of the State of Israel. Service of process upon us and upon our directors and officers and the Israeli experts named in this prospectus,
substantially all of whom reside outside the United States, may be difficult to obtain within the United States. Furthermore, because substantially all of our assets and substantially all of our directors and officers are located outside the United
States, any judgment obtained in the United States against us or any of our directors and officers may not be collectible within the United States.
We have irrevocably appointed Sol-Gel Technologies Inc. as our agent to receive service of process in any action against us in any U.S. federal or state court arising out of this
offering or any purchase or sale of securities in connection with this offering. The address of our agent is Sol-Gel Technologies Inc., c/o The Corporation Trust Center, 1209 Orange Street, Wilmington, Delaware 19801.
We have been informed by our legal counsel in Israel, Gross & Co., that it may be difficult to initiate an action with respect to U.S. securities law in Israel. Israeli
courts may refuse to hear a claim based on an alleged violation of U.S. securities laws reasoning that Israel is not the most appropriate forum to hear such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine
that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact by expert witnesses which can be a time-consuming and costly process. Certain matters
of procedure may also be governed by Israeli law.
Subject to certain time limitations and legal procedures and certain exceptions, Israeli courts may enforce a U.S. judgment in a civil matter which is non-appealable, including
judgments based upon the civil liability provisions of the Securities Act and the Exchange Act and including a monetary or compensatory judgment in a non-civil matter, provided that:
| • |
the judgment was rendered by a court which was, according to the laws of the state of the court, competent to render the judgment;
|
S - 26
| • |
the obligation imposed by the judgment is enforceable according to the rules relating to the enforceability of judgments in Israel and the substance of the judgment is not contrary to public policy; and
|
| • |
the judgment is executory in the state in which it was given.
|
Even if these conditions are met, an Israeli court will not declare a foreign civil judgment enforceable if:
| • |
the judgment was given in a state whose laws do not provide for the enforcement of judgments of Israeli courts (subject to exceptional cases);
|
| • |
the enforcement of the judgment is likely to prejudice the sovereignty or security of the State of Israel;
|
| • |
the judgment was obtained by fraud;
|
| • |
the opportunity given to the defendant to bring its arguments and evidence before the court was not reasonable in the opinion of the Israeli court;
|
| • |
the judgment was rendered by a court not competent to render it according to the laws of private international law as they apply in Israel;
|
| • |
the judgment is contradictory to another judgment that was given in the same matter between the same parties and that is still valid; or
|
| • |
at the time the action was brought in the foreign court, a lawsuit in the same matter and between the same parties was pending before a court or tribunal in Israel.
|
If a foreign judgment is enforced by an Israeli court, it generally will be payable in Israeli currency, which can then be converted into non-Israeli currency and transferred out
of Israel. Under existing Israeli law, a foreign judgment payable in foreign currency may be paid in Israeli currency at the rate of exchange in force on the date of the payment. Current Israeli exchange control regulations also permit a judgment
debtor to make payment in foreign currency. Pending collection, the amount of the judgment of an Israeli court stated in Israeli currency ordinarily will be linked to the Israeli consumer price index plus interest at the annual statutory rate set
by Israeli regulations prevailing at the time. Judgment creditors must bear the risk of unfavorable exchange rates.
S - 27
PROSPECTUS
$120,000,000
Ordinary Shares,
Warrants to Purchase Ordinary Shares,
Subscription Rights and/or Units
Offered by the Company

SOL-GEL TECHNOLOGIES LTD.
We may offer and sell to the public from time to time in one or more series or issuances up to $120,000,000 in the aggregate of ordinary shares, warrants, subscription rights
and/or units consisting of two or more of these classes or series of securities.
We refer to the ordinary shares, warrants, subscription rights and units collectively as “securities” in this prospectus.
Each time we sell securities pursuant to this prospectus, we will provide a supplement to this prospectus that contains specific information about the offeror, the offering
and the specific terms of the securities offered. This prospectus may not be used to consummate a sale of securities by us unless accompanied by the applicable prospectus supplement. You should read this prospectus and the applicable prospectus
supplement carefully before you invest in our securities.
We may, from time to time, offer to sell the securities, through public or private transactions, directly or through underwriters, agents or dealers, on or off the Nasdaq
Global Market, as applicable, at prevailing market prices or at privately negotiated prices. If any underwriters, agents or dealers are involved in the sale of any of these securities, the applicable prospectus supplement will set forth the
names of the underwriter, agent or dealer and any applicable fees, commissions or discounts.
Our ordinary shares are traded on the Nasdaq Global Market under the symbol “SLGL.” The last reported sale price for ordinary shares on April 4, 2022 as quoted on the Nasdaq
Global Market was $7.21 per share.
As of April 4, 2022, the aggregate market value worldwide of our outstanding voting and non-voting common equity held by non-affiliates was approximately $71.6 million, based
on 23,126,804 ordinary shares outstanding, of which 8,972,240 ordinary shares were held by non-affiliates, and a per ordinary share price of $7.98 based on the closing sale price of our ordinary shares on the Nasdaq Global Market on February 9,
2022. Pursuant to General Instruction I.B.5 of Form F-3, in no event will we sell, pursuant to the registration statement of which this prospectus supplement forms a part, securities with a value exceeding one-third of the aggregate market
value of our outstanding ordinary shares held by non-affiliates in any 12-month period, so long as the aggregate market value of our ordinary shares held by non-affiliates is less than $75.0 million. We have not offered or sold any securities
pursuant to General Instruction I.B.5 on Form F-3 during the prior 12 calendar month period that ends on and includes the date of this prospectus.
Investing in these securities involves a high degree of risk. Please carefully consider the risks discussed in this prospectus under “Risk Factors”
beginning on page 3 and the “Risk Factors” in “Item 3: Key Information- Risk Factors” of our most recent Annual Report on Form 20-F incorporated by reference in this prospectus and in any applicable prospectus supplement for a discussion of the
factors you should consider carefully before deciding to purchase these securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of the securities being offered by this
prospectus, or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2022
TABLE OF CONTENTS
Page
| 1 |
|
| 2 |
|
| 3 |
|
| 3 |
|
| 3 |
|
| 4 |
|
| 4 |
|
| 4 |
|
| 5 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 10 |
|
| 10 |
|
| 10 |
|
| 11 |
|
| 11 |
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission, or SEC, utilizing a “shelf” registration process. Under this
process, we may offer and sell our securities under this prospectus.
Under this shelf process, we may sell the securities described in this prospectus in one or more offerings up to a total price to the public of $120,000,000. The offer and
sale of securities under this prospectus may be made from time to time, in one or more offerings, in any manner described under the section in this prospectus entitled “Plan of Distribution.”
This prospectus provides you with a general description of the securities we may offer. Each time we sell securities we will provide a prospectus supplement that will contain
specific information about the terms of that offering. The prospectus supplement may also add, update or change information contained in this prospectus, and may also contain information about any material federal income tax considerations
relating to the securities covered by the prospectus supplement. You should read both this prospectus and any prospectus supplement together with additional information under the headings “Where You Can Find More Information” and “Incorporation
of Certain Documents by Reference.”
This summary may not contain all of the information that may be important to you. You should read this entire prospectus, including the financial statements and related notes
and other financial data incorporated by reference in this prospectus, before making an investment decision. This summary contains forward-looking statements that involve risks and uncertainties. Our actual results may differ significantly from
the results discussed in the forward-looking statements. Factors that might cause or contribute to such differences include those discussed in “Risk Factors” and “Forward-Looking Statements.”
1
Overview
We are a dermatology company focused on identifying, developing and commercializing investigational and generic topical drug products for the treatment
of skin diseases. In addition to Twyneo®, which has been approved by the FDA, our current product candidate pipeline consists of clinical stage and early-stage
investigational product candidates, some of which leverage our development platform, and several generic product candidates across multiple indications.
Our FDA-approved product, Twyneo®, is a novel, once-daily,
non-antibiotic topical cream containing a fixed-dose combination of encapsulated benzoyl peroxide and encapsulated tretinoin, that we developed for the treatment of acne vulgaris, or acne.
Our investigational product candidate, Epsolay®, is a novel, once-daily investigational topical cream containing encapsulated benzoyl peroxide, that we
are developing for the treatment of papulopustular (subtype II) rosacea.
In June 2021, we entered into two five-year exclusive license agreements with Galderma Holding SA (“Galderma”) pursuant to which Galderma
has the exclusive right to, and is responsible for, all U.S. commercial activities for Twyneo®, and, if approved by the FDA, Epsolay®.
Other investigational product candidates are SGT-210 that we are developing for the treatment of various keratodermas; SGT-310, an investigational aryl
hydrocarbon receptor agonist; and SGT-510.
We designed our proprietary, silica-based microencapsulation technology platform to enhance the tolerability and stability of topical drugs while
maintaining their efficacy. Topical drugs often struggle to balance achieving both high efficacy and high tolerability. Our technology platform entraps active ingredients in an inert, inorganic silica shell, which creates an unnoticeable
barrier between the active ingredient and the skin. The resulting microcapsules are designed to allow the entrapped active ingredients to gradually migrate through the pores of the shell and deliver active ingredient doses onto the skin in a
controlled manner, resulting in improved tolerability and stability without sacrificing efficacy. By separately encapsulating active ingredients within protective silica shells, our technology platform also enables the production of novel
fixed-dose active ingredient combinations that otherwise would not be stable. We believe that our microencapsulation technology has the potential to be used for topical drug products to treat a variety of skin diseases. As a result of the FDA
having already approved silica as a safe excipient for topical drug products, Both Twyneo® and Epsolay® were submitted for approval through the FDA’s 505(b)(2) regulatory pathway.
Corporate Information
Our legal and commercial name is Sol-Gel Technologies Ltd. We were incorporated on October 28, 1997 and were registered as a private company limited by shares under the
laws of the State of Israel. Our ordinary shares are traded on the Nasdaq Global Market under the symbol "SLGL".
Our principal executive offices are located at 7 Golda Meir St., Weizmann Science Park, Ness Ziona, 7403650 Israel, and our telephone number is
972-8-931-3433. Our website address is http://www.sol-gel.com. The information on our website does not constitute a part of this prospectus. Our agent for service of process in the United States is Sol-Gel Technologies Inc., c/o The
Corporation Trust Company, located at Corporation Trust Center, 1209 Orange Street, Wilmington, Delaware 19801.
2
An investment in our securities involves a high degree of risk. Our business, financial condition or results of operations could be adversely affected by any of these risks.
You should carefully consider the risk factors discussed under the caption "Item 3: Key Information - Risk Factors" in our Annual Report on Form 20-F for the year ended December 31, 2021, and in any
other filing we make with the SEC subsequent to the date of this prospectus, each of which are incorporated herein by reference, and in any supplement to this prospectus, before making your investment decision. The risks and uncertainties we
have described are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our operations. Past financial performance may not be a reliable indicator of future
performance, and historical trends should not be used to anticipate results or trends in future periods. If any of these risks actually occurs, our business, business prospects, financial condition or results of operations could be seriously
harmed. This could cause the trading price of our ordinary shares to decline, resulting in a loss of all or part of your investment. Please also read carefully the section below entitled “Forward-Looking Statements.”
We may sell from time to time pursuant to this prospectus (as may be detailed in a prospectus supplement) an indeterminate number of ordinary shares,
warrants to purchase ordinary shares, subscription rights and/or units comprised of any of the foregoing securities as shall have a maximum aggregate offering price of $120 million. The actual price per share or per security of the securities
that we will offer pursuant hereto will depend on a number of factors that may be relevant as of the time of offer. See “Plan of Distribution.”
We make forward-looking statements in this prospectus that are subject to risks and uncertainties. These forward-looking statements include information about possible or
assumed future results of our business, financial condition, results of operations, liquidity, plans and objectives. In some cases, you can identify forward-looking statements by terminology such as “believe,” “may,” “estimate,” “continue,”
“anticipate,” “intend,” “should,” “plan,” “expect,” “predict,” “potential,” or the negative of these terms or other similar expressions. Forward-looking statements are based on information we have when those statements are made or our
management’s good faith belief as of that time with respect to future events, and are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in or suggested by the
forward-looking statements. Important factors that could cause such differences include, but are not limited to:
| • |
the adequacy of our financial and other resources, particularly in light of our history of recurring losses and the uncertainty regarding the adequacy of our liquidity to pursue our complete business
objectives;
|
| • |
our ability to complete the development of our investigational product candidates;
|
| • |
our dependance on the success of Galderma in commercializing Twyneo® and Epsolay®;
|
| • |
the possibility that Galderma may terminate the collaboration agreement with respect to Epsolay® since Epsolay® was not approved for marketing by the FDA by March 31,
2022;
|
| • |
our ability to find suitable co-development, contract manufacturing and marketing partners;
|
| • |
our ability to obtain and maintain regulatory approvals for our investigational product candidates in our target markets and the possibility of adverse regulatory or legal actions relating to our
investigational product candidates even if regulatory approval is obtained;
|
| • |
our ability to commercialize and launch our pharmaceutical investigational product candidates;
|
| • |
our ability to obtain and maintain adequate protection of our intellectual property;
|
| • |
our ability to manufacture our investigational product candidates in commercial quantities, at an adequate quality or at an acceptable cost;
|
| • |
acceptance of Twyneo®, Epsolay® and our other investigational product candidates by healthcare professionals and patients;
|
| • |
the possibility that we may face third-party claims of intellectual property infringement;
|
3
| • |
the timing and results of clinical trials that we may conduct or that our competitors and others may conduct relating to our or their products;
|
| • |
intense competition in our industry, with competitors having substantially greater financial, technological, research and development, regulatory and clinical, manufacturing, marketing and sales,
distribution and personnel resources than we do;
|
| • |
potential product liability claims;
|
| • |
potential adverse federal, state and local government regulation in the United States, Europe or Israel;
|
| • |
the impact of ongoing pandemics such as Novel Coronavirus Disease 2019, or COVID-19, on our business and financial condition; and
|
| • |
loss or retirement of key executives and research scientists.
|
You should review carefully the risks and uncertainties described under the heading “Risk Factors” in this prospectus for a discussion of these and other risks that relate to
our business and investing in our ordinary shares. The forward-looking statements contained in this prospectus are expressly qualified in their entirety by this cautionary statement. Except as required by law, we undertake no obligation to
update publicly any forward-looking statements after the date of this prospectus to conform these statements to actual results or to changes in our expectations.
The table below sets forth our total capitalization as of December 31, 2021. The financial data in the following table should be read in conjunction with our financial
statements and notes thereto incorporated by reference herein.
|
|
As of December 31,
2021 |
|||
|
|
(in thousands)
|
|||
|
|
||||
|
Ordinary shares, par value NIS 0.1 per share
|
$
|
638
|
||
|
Additional paid-in capital
|
233,098
|
|||
|
Accumulated deficit
|
(178,142
|
)
|
||
|
Total shareholders’ equity
|
55,594
|
|||
|
Total capitalization
|
$
|
55,594
|
||
Our Ordinary Shares have been traded on the Nasdaq Global Market under the symbol “SLGL” from February 1, 2018.
Unless otherwise indicated in an accompanying prospectus supplement, the net proceeds from the sale of securities will be used for general corporate purposes, including
research and development related purposes in connection with our product candidates and for commercialization of our product candidates.
4
Our authorized share capital consists of 50,000,000 ordinary shares, par value NIS 0.1 per share, of which 23,126,804 shares were issued and outstanding as of December 31,
2021.
All of our outstanding ordinary shares will be validly issued, fully paid and non-assessable. Our ordinary shares are not redeemable and do not have any preemptive rights.
For a further description of our ordinary shares can be found under the heading "Description of Registrant’s Securities to be Registered" in our Registration Statement on
Form 8-A as filed with the SEC on January 26, 2018.
We may issue warrants to purchase ordinary shares. Warrants may be issued independently or together with any other securities and may be attached to, or separate from, such
securities. Each series of warrants will be issued under a separate warrant agreement to be entered into between us and a warrant agent. The warrant agent will act solely as our agent and will not assume any obligation or relationship of agency
for or with holders or beneficial owners of warrants. The terms of any warrants to be issued and a description of the material provisions of the applicable warrant agreement will be set forth in the applicable prospectus supplement.
The applicable prospectus supplement will describe the following terms of any warrants in respect of which this prospectus is being delivered:
|
•
|
the title of such warrants;
|
|
•
|
the aggregate number of such warrants;
|
|
•
|
the price or prices at which such warrants will be issued and exercised;
|
|
•
|
the currency or currencies in which the price of such warrants will be payable;
|
|
•
|
the securities purchasable upon exercise of such warrants;
|
|
•
|
the date on which the right to exercise such warrants shall commence and the date on which such right shall expire;
|
|
•
|
if applicable, the minimum or maximum amount of such warrants which may be exercised at any one time;
|
|
•
|
if applicable, the designation and terms of the securities with which such warrants are issued and the number of such warrants issued with each such security;
|
|
•
|
if applicable, the date on and after which such warrants and the related securities will be separately transferable;
|
|
•
|
information with respect to book-entry procedures, if any;
|
|
•
|
any material Israeli and United States federal income tax consequences;
|
|
•
|
the anti-dilution provisions of the warrants, if any; and
|
|
•
|
any other terms of such warrants, including terms, procedures and limitations relating to the exchange and exercise of such warrants.
|
Amendments and Supplements to Warrant Agreement
We and the warrant agent may amend or supplement the warrant agreement for a series of warrants without the consent of the holders of the warrants issued thereunder to effect
changes that are not inconsistent with the provisions of the warrants and that do not materially and adversely affect the interests of the holders of the warrants.
5
We may issue subscription rights to purchase our ordinary shares. These subscription rights may be issued independently or together with any other security offered hereby and
may or may not be transferable by the shareholder receiving the subscription rights in such offering. In connection with any offering of subscription rights, we may enter into a standby arrangement with one or more underwriters or other
purchasers pursuant to which the underwriters or other purchasers may be required to purchase any securities remaining unsubscribed for after such offering.
The prospectus supplement relating to any subscription rights we offer, if any, will, to the extent applicable, include specific terms relating to the offering, including
some or all of the following:
|
•
|
the price, if any, for the subscription rights;
|
|
•
|
the exercise price payable for each ordinary share upon the exercise of the subscription rights;
|
|
•
|
the number of subscription rights to be issued to each shareholder;
|
|
•
|
the number and terms of the ordinary shares which may be purchased per each subscription right;
|
|
•
|
the extent to which the subscription rights are transferable;
|
|
•
|
any other terms of the subscription rights, including the terms, procedures and limitations relating to the exchange and exercise of the subscription rights;
|
|
•
|
the date on which the right to exercise the subscription rights shall commence, and the date on which the subscription rights shall expire;
|
|
•
|
the extent to which the subscription rights may include an over-subscription privilege with respect to unsubscribed securities; and
|
|
•
|
if applicable, the material terms of any standby underwriting or purchase arrangement which may be entered into by us in connection with the offering of subscription rights.
|
The description in the applicable prospectus supplement of any subscription rights we offer will not necessarily be complete and will be qualified in its entirety by
reference to the applicable subscription right agreement, which will be filed with the SEC if we offer subscription rights. For more information on how you can obtain copies of the applicable subscription right agreement if we offer
subscription rights, see “Where You Can Find More Information; Incorporation of Information by Reference” beginning on page 10. We urge you to read the applicable subscription right agreement and any applicable prospectus supplement in their
entirety.
6
We may issue units comprised of one or more of the other securities that may be offered under this prospectus, in any combination. Each unit will be issued so that the holder
of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations of a holder of each included security. The unit agreement under which a unit is issued may provide that the
securities included in the unit may not be held or transferred separately at any time, or at any time before a specified date.
The prospectus supplement relating to any units we offer, if any, will, to the extent applicable, include specific terms relating to the offering, including some or all of
the following:
|
•
|
the material terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately;
|
|
•
|
any material provisions relating to the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units; and
|
|
•
|
any material provisions of the governing unit agreement that differ from those described above.
|
The description in the applicable prospectus supplement of any units we offer will not necessarily be complete and will be qualified in its entirety by reference to the
applicable unit agreement, which will be filed with the SEC if we offer units. For more information on how you can obtain copies of the applicable unit agreement if we offer units, see “Where You Can Find More Information; Incorporation of
Information by Reference” beginning on page 10. We urge you to read the applicable unit agreement and any applicable prospectus supplement in their entirety.
7
The securities being offered by this prospectus may be sold:
|
•
|
through agents;
|
|
•
|
to or through one or more underwriters on a firm commitment or agency basis;
|
|
•
|
through put or call option transactions relating to the securities;
|
|
•
|
through broker-dealers;
|
|
•
|
directly to purchasers, through a specific bidding or auction process, on a negotiated basis or otherwise;
|
|
•
|
through any other method permitted pursuant to applicable law; or
|
|
•
|
through a combination of any such methods of sale.
|
At any time a particular offer of the securities covered by this prospectus is made, a revised prospectus or prospectus supplement, if required, will be distributed which
will set forth the aggregate amount of securities covered by this prospectus being offered and the terms of the offering, including the name or names of any underwriters, dealers, brokers or agents, any discounts, commissions, concessions and
other items constituting compensation from us and any discounts, commissions or concessions allowed or reallowed or paid to dealers. Such prospectus supplement, and, if necessary, a post-effective amendment to the registration statement of
which this prospectus is a part, will be filed with the SEC to reflect the disclosure of additional information with respect to the distribution of the securities covered by this prospectus. In order to comply with the securities laws of
certain states, if applicable, the securities sold under this prospectus may only be sold through registered or licensed broker-dealers. In addition, in some states the securities may not be sold unless they have been registered or qualified
for sale in the applicable state or an exemption from registration or qualification requirements is available and is complied with.
The distribution of securities may be effected from time to time in one or more transactions, including block transactions and transactions on the Nasdaq Global Market or any
other organized market where the securities may be traded. The securities may be sold at a fixed price or prices, which may be changed, or at market prices prevailing at the time of sale, at prices relating to the prevailing market prices or at
negotiated prices. The consideration may be cash or another form negotiated by the parties. Agents, underwriters or broker-dealers may be paid compensation for offering and selling the securities. That compensation may be in the form of
discounts, concessions or commissions to be received from us or from the purchasers of the securities. Any dealers and agents participating in the distribution of the securities may be deemed to be underwriters, and compensation received by
them on resale of the securities may be deemed to be underwriting discounts. If any such dealers or agents were deemed to be underwriters, they may be subject to statutory liabilities under the Securities Act.
Agents may from time to time solicit offers to purchase the securities. If required, we will name in the applicable prospectus supplement any agent involved in the offer or
sale of the securities and set forth any compensation payable to the agent. Unless otherwise indicated in the prospectus supplement, any agent will be acting on a best efforts basis for the period of its appointment. Any agent selling the
securities covered by this prospectus may be deemed to be an underwriter, as that term is defined in the Securities Act, of the securities.
If underwriters are used in a sale, securities will be acquired by the underwriters for their own account and may be resold from time to time in one or more transactions,
including negotiated transactions, at a fixed public offering price or at varying prices determined at the time of sale, or under delayed delivery contracts or other contractual commitments. Securities may be offered to the public either
through underwriting syndicates represented by one or more managing underwriters or directly by one or more firms acting as underwriters. If an underwriter or underwriters are used in the sale of securities, an underwriting agreement will be
executed with the underwriter or underwriters, as well as any other underwriter or underwriters, with respect to a particular underwritten offering of securities, and will set forth the terms of the transactions, including compensation of the
underwriters and dealers and the public offering price, if applicable. The prospectus and prospectus supplement will be used by the underwriters to resell the securities.
If a dealer is used in the sale of the securities, we or an underwriter will sell the securities to the dealer, as principal. The dealer may then resell the securities to the
public at varying prices to be determined by the dealer at the time of resale. To the extent required, we will set forth in the prospectus supplement the name of the dealer and the terms of the transactions.
8
We may directly solicit offers to purchase the securities and may make sales of securities directly to institutional investors or others. These persons may be deemed to be
underwriters within the meaning of the Securities Act with respect to any resale of the securities. To the extent required, the prospectus supplement will describe the terms of any such sales, including the terms of any bidding or auction
process, if used.
Agents, underwriters and dealers may be entitled under agreements which may be entered into with us to indemnification by us against specified liabilities, including
liabilities incurred under the Securities Act, or to contribution by us to payments they may be required to make in respect of such liabilities. If required, the prospectus supplement will describe the terms and conditions of the
indemnification or contribution. Some of the agents, underwriters or dealers, or their affiliates may be customers of, engage in transactions with or perform services for us or our subsidiaries.
Any person participating in the distribution of securities registered under the registration statement that includes this prospectus will be subject to applicable provisions
of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the applicable SEC rules and regulations, including, among others, Regulation M, which may limit the timing of purchases and sales of any of our securities by that
person. Furthermore, Regulation M may restrict the ability of any person engaged in the distribution of our securities to engage in market-making activities with respect to our securities. These restrictions may affect the marketability of our
securities and the ability of any person or entity to engage in market-making activities with respect to our securities.
Certain persons participating in an offering may engage in over-allotment, stabilizing transactions, short-covering transactions, penalty bids and other transactions that
stabilize, maintain or otherwise affect the price of the offered securities. These activities may maintain the price of the offered securities at levels above those that might otherwise prevail in the open market, including by entering
stabilizing bids, effecting syndicate covering transactions or imposing penalty bids, each of which is described below.
|
•
|
A stabilizing bid means the placing of any bid, or the effecting of any purchase, for the purpose of pegging, fixing or maintaining the price of a security.
|
|
•
|
A syndicate covering transaction means the placing of any bid on behalf of the underwriting syndicate or the effecting of any purchase to reduce a short position created in connection
with the offering.
|
|
•
|
A penalty bid means an arrangement that permits the managing underwriter to reclaim a selling concession from a syndicate member in connection with the offering when offered securities
originally sold by the syndicate member are purchased in syndicate covering transactions.
|
These transactions may be effected on an exchange or automated quotation system, if the securities are listed on that exchange or admitted for trading on that automated
quotation system, or in the over-the-counter market or otherwise.
If so indicated in the applicable prospectus supplement, we will authorize agents, underwriters or dealers to solicit offers from certain types of institutions to purchase
offered securities from us at the public offering price set forth in such prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. Such contracts will be subject only to
those conditions set forth in the prospectus supplement and the prospectus supplement will set forth the commission payable for solicitation of such contracts.
In addition, ordinary shares may be issued upon conversion of or in exchange for debt securities or other securities.
Any underwriters to whom offered securities are sold for public offering and sale may make a market in such offered securities, but such underwriters will not be obligated to
do so and may discontinue any market making at any time without notice. The offered securities may or may not be listed on a national securities exchange. No assurance can be given that there will be a market for the offered securities.
Any securities that qualify for sale pursuant to Rule 144 or Regulation S under the Securities Act, may be sold under Rule 144 or Regulation S rather than pursuant to this
prospectus.
To the extent that we make sales to or through one or more underwriters or agents in at-the-market offerings, we will do so pursuant to the terms of a distribution agreement
between us and the underwriters or agents. If we engage in at-the-market sales pursuant to a distribution agreement, we will sell our ordinary shares to or through one or more underwriters or agents, which may act on an agency basis or on a
principal basis. During the term of any such agreement, we may sell ordinary shares on a daily basis in exchange transactions or otherwise as we agree with the underwriters or agents. The distribution agreement will provide that any ordinary
shares sold will be sold at prices related to the then prevailing market prices for our ordinary shares. Therefore, exact figures regarding proceeds that will be raised or commissions to be paid cannot be determined at this time and will be
described in a prospectus supplement. Pursuant to the terms of the distribution agreement, we also may agree to sell, and the relevant underwriters or agents may agree to solicit offers to purchase, blocks of our ordinary shares or warrants.
The terms of each such distribution agreement will be set forth in more detail in a prospectus supplement to this prospectus.
9
In connection with offerings made through underwriters or agents, we may enter into agreements with such underwriters or agents pursuant to which we receive our outstanding
securities in consideration for the securities being offered to the public for cash. In connection with these arrangements, the underwriters or agents may also sell securities covered by this prospectus to hedge their positions in these
outstanding securities, including in short sale transactions. If so, the underwriters or agents may use the securities received from us under these arrangements to close out any related open borrowings of securities.
We may enter into derivative transactions with third parties or sell securities not covered by this prospectus to third parties in privately negotiated transactions. If the
applicable prospectus supplement indicates, in connection with those derivatives, such third parties (or affiliates of such third parties) may sell securities covered by this prospectus and the applicable prospectus supplement, including in
short sale transactions. If so, such third parties (or affiliates of such third parties) may use securities pledged by us or borrowed from us or others to settle those sales or to close out any related open borrowings of shares, and may use
securities received from us in settlement of those derivatives to close out any related open borrowings of shares. The third parties (or affiliates of such third parties) in such sale transactions will be underwriters and, if not identified in
this prospectus, will be identified in the applicable prospectus supplement (or a post-effective amendment).
We may loan or pledge securities to a financial institution or other third party that in turn may sell the securities using this prospectus. Such financial institution or
third party may transfer its short position to investors in our securities or in connection with a simultaneous offering of other securities offered by this prospectus or in connection with a simultaneous offering of other securities offered by
this prospectus.
Certain legal matters with respect to Israeli law and with respect to the validity of the offered securities under Israeli law will be passed upon for us by Gross & Co. Certain legal matters with respect to U.S. law will be passed upon for us by Latham & Watkins LLP, New York, New York.
The financial statements incorporated in this prospectus by reference to the Annual Report on Form 20-F for the year ended December 31, 2021 have been so incorporated in
reliance on the report of Kesselman & Kesselman, Certified Public Accountants (Isr.), a member firm of PricewaterhouseCoopers International Limited, an independent registered public accounting firm, given on the authority of said firm as
experts in auditing and accounting.
We have filed with the SEC a registration statement on Form F-3 under the Securities Act, with respect to the securities offered by this prospectus. However, as is permitted
by the rules and regulations of the SEC, this prospectus, which is part of our registration statement on Form F-3, omits certain non-material information, exhibits, schedules and undertakings set forth in the registration statement. For further
information about us, and the securities offered by this prospectus, please refer to the registration statement.
We are subject to the reporting requirements of the Exchange Act that are applicable to a foreign private issuer. In accordance with the Exchange Act, we file reports,
including annual reports on Form 20-F by April 30 of each year. We also furnish to the SEC under cover of Form 6-K material information required to be made public in Israel, filed with and made public by any stock exchange or distributed by us
to our shareholders.
The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers, such as us, that file electronically with
the SEC (http://www.sec.gov).
As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements to shareholders and our officers,
directors and principal shareholders are exempt from the “short-swing profits” reporting and liability provisions contained in Section 16 of the Exchange Act and related Exchange Act rules.
10
We file annual and special reports and other information with the SEC (File Number 001-38367). These filings contain important information which does not appear in this
prospectus. The SEC allows us to “incorporate by reference” information into this prospectus, which means that we can disclose important information to you by referring you to other documents which we have filed or will file with the SEC. We
are incorporating by reference in this prospectus the documents listed below and all amendments or supplements we may file to such documents, as well as any future filings we may make with the SEC on Form 20-F under the Exchange Act before the
time that all of the securities offered by this prospectus have been sold or de-registered:
| • |
our Annual Report on Form 20-F for the fiscal year
ended on December 31, 2021, filed with the SEC on April 4, 2022, as amended by Amendment No. 1 to Form 20-F, filed with the SEC on April 7, 2022; and
|
| • |
the description of our ordinary shares contained under the heading “Item 1. Description of Registrant’s Securities to be Registered” in our registration statement on Form 8-A, as filed with the SEC on January 26, 2018, including any subsequent amendment or any report filed for the purpose of updating
such description.
|
In addition, any reports on Form 6-K submitted to the SEC by the registrant pursuant to the Exchange Act after the date of the initial registration statement and prior to effectiveness of the
registration statement that we specifically identify in such forms as being incorporated by reference into the registration statement of which this prospectus forms a part and all subsequent annual reports on Form 20-F filed after the effective
date of this registration statement and prior to the termination of this offering and any reports on Form 6-K subsequently submitted to the SEC or portions thereof that we specifically identify in such forms as being incorporated by reference
into the registration statement of which this prospectus forms a part, shall be considered to be incorporated into this prospectus by reference and shall be considered a part of this prospectus from the date of filing or submission of such
documents.
Certain statements in and portions of this prospectus update and replace information in the above listed documents incorporated by reference. Likewise, statements in or
portions of a future document incorporated by reference in this prospectus may update and replace statements in and portions of this prospectus or the above listed documents.
We will provide you without charge, upon your written or oral request, a copy of any of the documents incorporated by reference in this prospectus, other than exhibits to
such documents which are not specifically incorporated by reference into such documents. Please direct your written or telephone requests to Sol-Gel Technologies Ltd., 7 Golda Meir Street, Weizmann Science Park, Ness Ziona, 7403650, Israel,
Attn: Gilad Mamlok, telephone number +972 (8) 931-3433. You may also obtain information about us by visiting our website at www.sol-gel.com. Information contained in our website is not part of this
prospectus.
We are incorporated under the laws of the State of Israel. Service of process upon us and upon our directors and officers and the Israeli experts named in this prospectus,
substantially all of whom reside outside the United States, may be difficult to obtain within the United States. Furthermore, because substantially all of our assets and substantially all of our directors and officers are located outside the
United States, any judgment obtained in the United States against us or any of our directors and officers may not be collectible within the United States.
We have irrevocably appointed Sol-Gel Technologies Inc. as our agent to receive service of process in any action against us in any U.S. federal or state court arising out of
this offering or any purchase or sale of securities in connection with this offering. The address of our agent is Corporation Trust Center, 1209 Orange Street, Wilmington,
Delaware 19801.
We have been informed by our legal counsel in Israel, Gross & Co., that it may be difficult to initiate an action with respect to U.S. securities law in Israel. Israeli
courts may refuse to hear a claim based on an alleged violation of U.S. securities laws reasoning that Israel is not the most appropriate forum to hear such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine
that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact by expert witnesses which can be a time-consuming and costly process. Certain
matters of procedure may also be governed by Israeli law.
11
Subject to certain time limitations and legal procedures, Israeli courts may enforce a U.S. judgment in a civil matter which, subject to certain exceptions, is
non-appealable, including judgments based upon the civil liability provisions of the Securities Act and the Exchange Act and including a monetary or compensatory judgment in a non-civil matter, provided that:
| • |
the judgment was rendered by a court which was, according to the laws of the state of the court, competent to render the judgment;
|
| • |
the obligation imposed by the judgment is enforceable according to the rules relating to the enforceability of judgments in Israel and the substance of the judgment is not contrary to public policy; and
|
| • |
the judgment is executory in the state in which it was given.
|
Even if these conditions are met, an Israeli court will not declare a foreign civil judgment enforceable if:
| • |
the judgment was given in a state whose laws do not provide for the enforcement of judgments of Israeli courts (subject to exceptional cases);
|
| • |
the enforcement of the judgment is likely to prejudice the sovereignty or security of the State of Israel;
|
| • |
the judgment was obtained by fraud;
|
| • |
the opportunity given to the defendant to bring its arguments and evidence before the court was not reasonable in the opinion of the Israeli court;
|
| • |
the judgment was rendered by a court not competent to render it according to the laws of private international law as they apply in Israel;
|
| • |
the judgment is contradictory to another judgment that was given in the same matter between the same parties and that is still valid; or
|
| • |
at the time the action was brought in the foreign court, a lawsuit in the same matter and between the same parties was pending before a court or tribunal in Israel.
|
If a foreign judgment is enforced by an Israeli court, it generally will be payable in Israeli currency, which can then be converted into non-Israeli currency and transferred
out of Israel. The usual practice in an action before an Israeli court to recover an amount in a non-Israeli currency is for the Israeli court to issue a judgment for the equivalent amount in Israeli currency at the rate of exchange in force on
the date of the judgment, but the judgment debtor may make payment in foreign currency. Pending collection, the amount of the judgment of an Israeli court stated in Israeli currency ordinarily will be linked to the Israeli consumer price index
plus interest at the annual statutory rate set by Israeli regulations prevailing at the time. Judgment creditors must bear the risk of unfavorable exchange rates.
12
