UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16
Under the Securities Exchange Act of 1934
For the month of February 2020
Commission File Number 001-38367
SOL-GEL TECHNOLOGIES LTD.
(Translation of registrant’s name into English)
7 Golda Meir Street
Ness Ziona 7403650, Israel
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
INFORMATION CONTAINED IN THIS REPORT ON FORM 6-K
Sol-Gel Technologies Ltd. (the “Company”) is posting on its website a corporate presentation.
Attached hereto and incorporated by reference in this Report on Form 6-K is the following exhibit:
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this
report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
SOL-GEL TECHNOLOGIES LTD.
|
|||
|
Date: February 5, 2020
|
By:
|
/s/ Gilad Mamlok | |
| Gilad Mamlok | |||
|
Chief Financial Officer
|
|||
Exhibit 99.1

NASDAQ: SLGL

FORWARD-LOOKING STATEMENTS This presentation contains forward-looking statements within the meaning of
the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,”
“expect,” “plan,” “anticipate,” “could,” “future,” “outlook,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential,” “continue,” or the negative of these terms or other similar expressions, although not all
forward-looking statements contain these words. The forward-looking statements in this presentation relate to, among other things, our anticipated NDA submission dates for Epsolay and Twyneo, estimated timing for the approval and launch of
Epsolay and Twyneo, and estimated sales of our product candidates. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties, and other important factors that may cause our actual results,
performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by the forward-looking statement, including but not limited to the following: the fact that we have and expect to
continue to incur significant losses; our need for additional funding, which may not be available; our ability to complete the development of our product candidates; our ability to obtain and maintain regulatory approvals for our product
candidates in our target markets and the possibility of adverse regulatory or legal actions relating to our product candidates even if regulatory approval is obtained; our ability to commercialize our product candidates; our ability to obtain
and maintain adequate protection of our intellectual property; our ability to manufacture our product candidates in commercial quantities, at an adequate quality or at an acceptable cost; our ability to establish adequate sales, marketing, and
distribution channels; acceptance of our product candidates by healthcare professionals and patients; the possibility that we may face third-party claims of intellectual property infringement; the timing and results of clinical trials that we
may conduct or that our competitors and others may conduct relating to our or their products; intense competition in our industry, with competitors having substantially greater financial, technological, research and development, regulatory and
clinical, manufacturing, marketing, and sales, distribution and personnel resources than we do; potential product liability claims; potential adverse federal, state, and local government regulation in the United States, Europe, or Israel; and
loss or retirement of key executives and research scientists. These and other important factors discussed in the Company's Annual Report on Form 20-F filed with the Securities and Exchange Commission (“SEC”) on March 21, 2019, and our other
reports filed with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this presentation. Any such forward-looking statements represent management’s estimates as of the date of
this presentation. While we may elect to update such forward-looking statements at some point in the future, unless required by applicable law, we disclaim any obligation to do so, even if subsequent events cause our views to change. Thus, one
should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements. These forward-looking statements should not be relied upon as representing our views as of any
date subsequent to the date of this presentation. This presentation contains trademarks, trade names, and service marks of other companies, which are the property of their respective owners. We do not intend our use or display of other parties'
trademarks, trade names, or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties.Before you invest, you should read the prospectus in
the registration statement and other documents we have filed with the SEC for more complete information about the issuer and this offering. You may get these documents for free by visiting EDGAR on the SEC web site at http://www.sec.gov.
Alternatively, we, any underwriter or any dealer participating in the offering will arrange to end you the prospectus if you request it from Jefferies, Attention Equity Syndicate Prospectus Department, 520 Madison Avenue, 2nd Floor, New York,
NY 10022, via telephone at (877) 821-7388, or email at: [email protected] or from BMO Capital Markets, Attention: Syndicate Department, 3 Times Square, 25th Floor, New York, New York 10036 or by telephone at (800) 414-3627 or
by email [email protected].

THREE-FOLD STRATEGY Leverage our capabilities to generate significant non-dilutive funding Identify
targeted opportunities in other areas of high unmet need where we can bring innovation and exceed current standard-of-care treatments Successfully commercialize best-in-class dermatology brands in acne and rosacea, and maintain a leadership
position in these indications

NOVEL DELIVERY SYSTEMFOR BEST-IN-CLASS TOPICAL DRUGS Proprietarysilica-based microencapsulation topical
delivery platform for dermatology indications Positive phase III results from EPSOLAY® clinical trial in papulopustular rosacea in July 2019NDA submission anticipated in 1H/2020 Positive phase III results from TWYNEO® in acne vulgaris in
December 2019NDA submission anticipated in 2H/2020 Completed follow-on offering of $11.5 million in August 2019Successfully raised $86.3 million in IPO in February 2018 Non-dilutive revenues of $18.8 million from generic pipeline in the first
9 months Seasoned management team with proven track record and broad dermatologic experience 1 2 3 4 5 6

Pipelines & upcoming milestones BRANDED CANDIDATES EPSOLAY®Papulopustular
rosacea TWYNEO®Acne vulgaris Research/Preclinical Phase I Phase II Phase III NDA Submission 1H/2020 NDA Submission 2H/2020 GENERIC PRODUCTS/CANDIDATES Ivermectin cream, 1%(RLD: Soolantra®) Acyclovir cream, 5%(RLD:
Zovirax®) 5-Fluorouracil cream, 5%(RLD: Efudex®) TENTATIVE APPROVAL AS OF JANUARY 29, 2018 APPROVAL & SALES AS OF FEBRUARY 2019 BIOEQUIVALENCE ACHIEVED IN DEC
2019 Research Filed Bioequivalence SGT-210Palmoplantar keratoderma Anticipated Milestones RLD, reference listed drug. Data in 1H/2021 TapinarofPsoriasis & other derm indications RoflumilastPsoriasis
& other derm indications Formulation Formulation

WHY SILICA? FDA approved for topical useProprietary process produces high encapsulation
efficiencyPhysical properties of silica shell tuned to modify release of active ingredientSmooth, no-grit feel for userStrong IP protection to 2032 (EPSOLAY®) and 2038 (TWYNEO®) 1 Silica monomers and drug substance are emulsified together
Silica monomers migrate to the oil/water interface in a well-controlled process A silica shell, microcapsuleis formed SOL-GEL PROCESS 2 Barrier between entrapped API and skin may reduce irritation and improve complianceAPIs stabilized
via microencapsulation, allowing for novel combinationsHurdle for generics to demonstrate similar release profileIf approved, will be first core-shell encapsulation system for topical dermatology products POTENTIAL BENEFITS 3 Foundation
forbranded product pipeline

CRYO-SEM PICTURE ENERGY-DISPERSIVE X-RAY SPECTROSCOPY MAPPING Encapsulated Benzoyl Peroxide
(E-BPO) Skin lipids migrate through the silica shell to promote solubilization of BPO. Dissolved BPO then migrates to skin’s sebaceous follicles Silica shell wraps BPO crystals and serves as a barrier between BPO crystals and skin, leading to
less irritation Controlled release improves tolerability

Patents and Trademarks # of Patents Related to Company Products 5 11 34 15 4 in US, IL, CA,
EP EPSOLAY® 5 in US, CA, EP, IL TWYNEO® US Patents Granted/Allowed Pending Foreign Patents Granted/Allowed Pending Trademarks Registered/Allowed Registered/Allowed Our intellectual property is protected through a series of patent
families, describing and claiming our proprietary processes, formulations, and methods of use IP, Expiry Product/Indication IP Protection for Our Branded Products (US) Granted 2038Pending 2040 Granted 2032Pending 2040 TWYNEO®acne
vulgaris EPSOLAY®subtype II rosacea Intellectual property estate

A multifactorial disease of the pilosebaceous unit, involving abnormalities in sebum production,
follicular epithelial desquamation, bacterial proliferation, and inflammation Topical BPO, retinoids, antibiotics, and their combinations; isotretinoin and antibiotics are mainstays of systemic therapy Insufficient efficacy negatively
affects self-esteem; contributes to antibiotic resistance; systemic side effects What isacne vulgaris? How is it treated? What are the current treatment shortfalls? Acne vulgaris Multifactorial disease requiring powerful combination
treatments Our solution: TWYNEO®E-BPO + E-ATRA Cream Encapsulation allows combining 2 highly effective APIs, BPO and ATRA, that have complementary mechanisms of actionEncapsulation may reduce the irritation of both BPO and ATRAPotential to
be more effective than existing topical treatments

Co-Primary EndpointsProportion of subjects with an assessment of clear or almost clear and with at least
a 2-grade improvement in IGA from baseline at Week 12Absolute change in inflammatory lesion counts from baseline at Week 12Absolute change in non-inflammatory lesion counts from baseline at Week 12Safety EndpointsCutaneous safety assessment,
local tolerability assessment, adverse event reporting QD, Self-applied Twyneo® cream(3% E-BPO, 0.1% E-ATRA) Vehicle cream Age ≥9 years≥20 to ≤100 Inflammatory lesions ≥30 to ≤150 Non-inflammatory lesionsIGA grade 3 (Moderate) or grade 4
(Severe)Cysts/nodules ≤2 2:1 Randomization Inclusion criteria 12 weeks of treatment E-ATRA=microencapsulated tretinoin; E-BPO=microencapsulated benzoyl peroxide; IGA=Investigator's Global Assessment; QD=once daily; Baseline 2 4 8 12
Weeks 63 Total SitesStudy 65-04: 424Study 65-05: 434 Twyne0® Study design Two Phase 3, Double-blind, Randomized, Vehicle-controlled Studies

Study 65-04 Study 65-05 Number of sites 32 31 Twyneo®(n=281) Vehicle (n=143)
Twyneo®(n=290) Vehicle (n=144) Age, yearsMean (SD)Median (range) 20.9 (8.48)18.0 (11-67) 21.4 (8.62)18.0 (10-57) 20.1 (6.96)18.0 (10-51) 20.3 (6.67)18.5 (9-42) Sex, n (%)MaleFemale 106 (37.7%)175 (62.3%) 60 (42.0%)83 (58.0%) 117
(40.3%)173 (59.7%) 67 (46.5%)77 (53.5%) Ethnicity, n (%)Hispanic/LatinoNot Hispanic or LatinoUnknown/Not Reported 102 (36.3%)178 (63.3%)1 (0.4%) 44 (30.8%)98 (68.5%)1 (0.7%) 85 (29.3%)204 (70.3%)1 (0.3%) 56 (38.9%)87 (60.4%)1 (0.7%) IGA
severity Moderate Severe 251 (89.3%)30 (10.7%) 132 (92.3%)11 (7.7%) 262 (90.3%)28 (9.7%) 133 (93.0%)10 (7.0%) Inflammatory lesion countMean (SD)Median (range) 33.5 (14.62)28.0 (20-92) 33.5 (14.69)28.0 (20-90) 28.2 (8.70)25.0
(20-62) 27.5 (8.52)25 (20-75) Non-inflammatory lesion countMean (SD)Median (range) 48.6 (20.24)42.0 (30-148) 47.1 (19.97)41.0 (30-140) 44.6 (18.03)39.0 (23-149) 44.9 (18.82)38.0 (30-123) Well-balanced studies at Baseline (ITT)

Low discontinuation rate across studies Study 65-04 Study 65-05 Twyneo®(n=281) Vehicle
(n=143) Twyneo®(n=290) Vehicle (n=144) Randomized Subjects Discontinued 32 12 48 12 Adverse events 4 (1.4%) 0 12 (4.1%) 0 Lost to follow-up 10 (3.6%) 7 (4.9%) 15 (5.2%) 7 (4.9%) Lack of
efficacy 0 0 0 0 Pregnancy 1 (0.4%) 0 1 (0.3%) 0 Protocol violation 2 (0.7%) 0 0 0 Withdrawal by parent/guardian 4 (1.4%) 1 (0.7%) 4 (1.4%) 0 Withdrawal by patient 9 (3.2%) 4 (2.8%) 14 (4.8%) 5
(3.5%) Physician decision 1 (0.4%) 0 1 (0.3%) 0 Condition worsened 0 0 0 0 Other 1 (0.4%) 0 1 (0.3%) 0 Completed 249 (88.6%) 131 (91.6%) 242 (83.4%) 132 (91.7%)

Co-primary endpoint (itt) IGA Treatment Success at Week 12 Percent of subjects achieving IGA success at
Week 12 P<0.001 Study 65-04 Vehicle(n=143) Vehicle P=0.017 Study 65-05 (n=290) (n=144) (n=281)

Co-primary endpoint (itt) Absolute Mean Change From Baseline in Inflammatory Lesions at Week
12 P<0.001 P=0.018 Study 65-04 Study 65-05 Mean reduction in inflammatory lesion count from baseline at Week 12 Vehicle(n=143) (n=281) (n=290) Vehicle(n=144)

Co-primary endpoint (itt) Absolute Mean Change From Baseline in Non-Inflammatory Lesions at Week
12 Mean reduction in non-inflammatory lesion count from baseline at Week 12 Study 65-04 Study 65-05 P<0.001 P<0.001 Vehicle(n=143) Vehicle(n=144) Twyneo®(n=281) Twyneo®(n=290)

SUCCESS IN IGA IN RECENT ACNE TRIALS* Trials With Highest Difference in IGA Between the Active Arm and
the Vehicle Arm *Sol-Gel did not conduct a head-to-head comparison trial or study. The results described above are for illustrative purposes only and should not be construed as conclusions to be drawn as if we conducted a head-to-head
comparison trial or study Percent of subjects achieving IGA success at Week 12 normalized to vehicle Twyneo® Winlevi® Seysara®
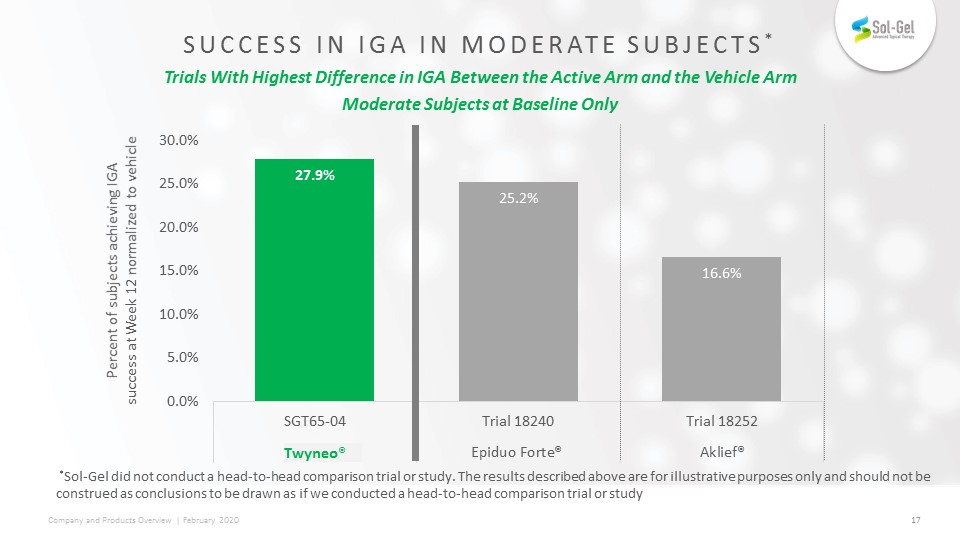
SUCCESS IN IGA IN MODERATE Subjects* Trials With Highest Difference in IGA Between the Active Arm and
the Vehicle ArmModerate Subjects at Baseline Only Percent of subjects achieving IGA success at Week 12 normalized to vehicle Twyneo® *Sol-Gel did not conduct a head-to-head comparison trial or study. The results described above are for
illustrative purposes only and should not be construed as conclusions to be drawn as if we conducted a head-to-head comparison trial or study

Twyneo®(n=281) Supportive efficacy analysis*(itt) IGA Treatment Success Over Time Percent of subjects
achieving IGA success Week Week Study 65-04 Study 65-05 *Percent of subjects with an assessment of clear or almost clear and with at least a 2-grade improvement in IGA from baseline, at Weeks 2, 4 and
8 Vehicle(n=143) Twyneo®(n=290) Vehicle(n=144)

Supportive efficacy analysis* (itt) Mean Reduction in Non-Inflammatory Lesion Count Over Time Mean
change from baseline in non-inflammatory lesion count Study 65-04 Study 65-05 Week Week Mean change from baseline in inflammatory lesion count Study 65-04 Study 65-05 Week Week Mean Reduction in Inflammatory Lesion Count Over
Time *Mean change from baseline in inflammatory and non-inflammatory lesion counts from baseline to Week 2 Twyneo® Twyneo®

Safety & tolerability Nearly all AEs were mild or moderate in severityTotal of 18 subjects
discontinued from Studies 65-04 and 65-05 due to a TEAE: 18 (2%) in Twyneo® and 0 in vehicle No treatment-related SAEs were identified in either study2 subjects reported SAEs in Study 65-05; (1) Twyneo® subject reported depression Study
65-04 Study 65-05 Most frequent non-cutaneous TEAEs (≥1% in any treatment arm), n (%) Twyneo® Vehicle Twyneo® Vehicle Safety population n=274 n=139 n=281 n=138 Upper respiratory tract infection 6 (2.2%) 3 (2.2%) 1 (0.4%) 2
(1.4%) Headache 3 (1.1%) 1 (0.7%) 1 (0.4%) 0 Nasopharyngitis 1 (0.4%) 0 4 (1.4%) 0 Attention deficithyperactivity disorder 0 2 (1.4%) 0 0 Viral upper respiratorytract infection 0 0 1 (0.4%) 2 (1.4%) SAE=serious adverse
event; TEAE=treatment-emergent adverse event

Local Skin Tolerability assessment* at week 12 *Safety population Study 65-04 Study 65-05

Local skin tolerability assessments over time Safety population for Study 65-04 (n=274). Safety
population for Study 65-04 (n=281). BL=baseline; 12W=12 weeks Erythema Scaling Pigmentation Dryness Itching Burning Stinging Percent of subjects BL 12W BL 12W BL 12W BL 12W BL 12W BL 12W BL 12W BL 12W BL 12W BL 12W BL 12W Study
65-05 Study 65-04 BL 12W BL 12W BL 12W

Encapsulation aims to reduce irritation of BPOPotential to be more effective than existing
treatmentsPotential to be first FDA-approved single-agent BPO Rx drug product Chronic, inflammatory condition that primarily affects the face and is often characterized by flushing, redness, inflamed bumps, and pustules Topical
antimicrobials (metronidazole, clindamycin); topical anti-mite (ivermectin); systemic antibiotics (minocycline, doxycycline) Insufficient efficacy resulting in poor adherence, contributing to antibiotic resistance; systemic side effects What
ispapulopustular rosacea? How is it treated? What are the current treatment shortfalls? Papulopustular rosacea Inflammatory condition with poor adherence to current treatments Our solution: EPSOLAY®Encapsulated benzoyl peroxide (E-BPO)

Male and female ≥18 years of age Clinical diagnosis of moderate to severe rosacea≥15 to ≤70 inflammatory
lesions≤2 nodules 2:1 EPSOLAY® cream, 5% (once daily) Vehicle cream (once daily) 54 Total SitesStudy 54-01: 361Study 54-02: 372 12 weeks of treatment Baseline 2 4 8 12 Weeks Inclusion criteria Two phase III, double-blind, randomized,
vehicle-controlled studies EPSOLAY® Study design PRIMARY ENDPOINTS:Proportion of patients with the primary measure of success, "Clear" (0) or "Almost clear" (1), in the Investigator GlobalAssessment (IGA) relative to baseline at Week
12Absolute mean change in inflammatory lesion counts from baseline to Week 12 Randomization

STUDY POPULATIONS & DISCONTINUATION Study 54-02 Study 54-01 Percent of
patients EPSOLAY® Vehicle Randomized (n=243), Safety (n=239),Per Protocol (n=190) Randomized (n=118), Safety (n=113),Per Protocol (n=93) Randomized (n=250), Safety (n=249),Per Protocol (n=235) Percent of patients Randomized (n=122),
Safety (n=120),Per Protocol (n=113) Intent-to-treat population.

Patient severity at baseline Study 54-01 Characteristic EPSOLAY® Vehicle IGA “Moderate”IGA
“Severe” 210 (86.4%)33 (13.6%) 104 (88.1%)14 (11.9%) Mean lesion count (SD)Median lesion count (range) 25.7 (11.07)22.0 (15-69) 26.3 (12.45)21.0 (15-70) Study 54-02 EPSOLAY® Vehicle 227 (90.8%)23 (9.2%) 112 (91.8%)10 (8.2%) 29.8
(14.00)25.0 (15-70) 27.5 (13.04)22.5 (15-70)

Primary endpoints (itt) Study 54-02 Study 54-01 P<0.001 P<0.001 Success in IGA at Week
12 Inflammatory Lesion Count Change From Baseline at Week 12 P<0.001 P<0.001 Study 54-02 Study 54-01 ITT, intent-to-treat.

Secondary endpoint (itt) Inflammatory Lesion Percent Change From Baseline to Week
12 P<0.001 P<0.001 Study 54-02 Study 54-01

Success in iga (itt) Week 2Exploratory Endpoint P=0.009 P=0.017 Study 54-02 Study 54-01 Week
4Secondary Endpoint P<0.001 P=0.009 Study 54-02 Study 54-01 Week 8Secondary Endpoint P<0.001 P=0.006 Study 54-02 Study 54-01

Inflammatory lesion Count Change from Baseline (itt) Study 54-02 Study
54-01 P<0.001 P<0.001 Week 2Exploratory Endpoint Week 4Secondary Endpoint Week 8Secondary Endpoint Study 54-02 Study 54-01 P<0.001 P<0.001 Study 54-02 Study 54-01 P<0.001 P<0.001

Comparison of Onset of action tohistorical soolantra® results* Rapid Onset of EPSOLAY® *Sol-Gel did not
conduct a head-to-head comparison trial or study. The results described above are for illustrative purposes only and should not be construedas conclusions to be drawn as if we conducted a head-to-head comparison trial or study.

Baseline Characteristics of Active
Arm IGA Severe 33 23 82 113 26 65 0 51 71 52 48 Moderate 210 227 369 346 172 418 557 444 443 67 77 Mild 0 0 0 0 0 0 0 0 0 8 17 Inflammatory
Lesions 25.7 29.8 31.0 33.3 21.6 21.7 18.3 28.5 30.0 19.5 20.5 Inflammatory Lesions–Mean Percent Change From Baseline Success in IGA Difference From Vehicle FMX103 Minocycline foam, 1.5% 10-week study EPSOLAY® Oral
administration 16-week study Side-by-side with other historicaltrial results* *Sol-Gel did not conduct a head-to-head comparison trial or study. The results described above are for illustrative purposes only and should not be construedas
conclusions to be drawn as if we conducted a head-to-head comparison trial or study. 12-week study 12-week study 12-week study 12-week study

SKIN TOLERABILITY Safety population. Dryness Scaling Burning/Stinging Itching Percent Reporting Any
(%) Study 54-01 Percent Reporting Any (%) Study 54-02 Dryness Scaling Burning/Stinging Itching

Study 54-01 Study 54-02 TEAEs, n (%) EPSOLAY®(n=239) Vehicle(n=113) EPSOLAY®(n=249) Vehicle
(n=120) Any TEAE 49 (20.5%) 17 (15.0%) 50 (20.2%) 22 (18.2%) Serious TEAE 0 1 (0.4%)* 1 (0.4%)† 0 Severe TEAE 2 (0.8%) 0 2 (0.8%)‡ 0 Discontinuation 5 (2.1%) 1 (0.9%) 4 (1.6%) 1 (0.8%)§ Treatment-related 14 (5.9%) 3
(2.7%) 9 (3.6%) 0 *Femur fracture.†Spinal compression fracture.‡One subject with spinal compression fracture.§Urinary tract infection—Discontinuation classified as “other reason.” Safety population. Treatment-emergent adverse events(TEAEs)
summary

ROSACEA ACNE Approximately 16 million people in the US suffer from rosacea; 5-6 million have type 2
(age >30 years)~$800 million branded topical market (WAC)1Treated with topical products 76% of the time1Dermatologists account for 80% of treatmentsMany patients are misdiagnosed or do not seek treatment at all, creating a large underserved
patient population Market potential for acne & rosacea 1. Symphony Health. Syneos Research & Insights ”Treatment Answers”; June 2019 MAT. 50 million people suffer from acne in the US(ages 12-24 years)~$1.9 billion branded topical
market (WAC)1 Treated with topicals 56% of the time;remaining is oral1Dermatologists account for ~60% of acne treatments(higher for branded products)Combining treatments is the bestway to combat acne for the majority of patients2 2. American
Academy of Dermatology. https://www.aad.org/practicecenter/quality/clinical-guidelines/acne/topical-therapies..

EPSOLAY® Advanced technology platform Trusted API Topical creamNon-systemicAntibiotic-freeComplimentary
mechanism Potential to advance rosacea treatment Demonstrated strong efficacy Demonstrated fast onset of action Observed favorable tolerability profile

DENSITY &PRODUCTIVITY METRICS MARKET FACTORS APPROACH TO building a
commercialorganization—Efficient and effectivE PRESCRIBER VALUE ~12,000 Dermatologists ~6,500 Decile3-10 ~6,000 NP/PAs FlexibleScalableHighly efficient SALES FORCE 3,280 target offices~45-62 sales representatives

COMPETITIVE PRICING Addressing access & UM for epsolay®1-3 ~70% Most would cover at preferred or
non-preferred level dependingon cost PAYER RESPONSE TO CLINICAL PROFILE LIKELY:Step-through genericsQuantity limits POSSIBLE:Prior authorizationto label “If priced like Finacea, it would get parity access; 15%-20% rebate expected with
WAC at parity to Finacea.” State AIS Health, 2019. http://www.aishealth.com/about.MMIT Network, 2019. http://www.mmitnetwork.com.Data on file. NPG Health primary market research, 2019. PAYER UM POSITION BASED ON HIGHERNET-TO-PLAN PRICE
COMPELLING TO DRIVE FORMULARY CONSIDERATION COVERED OR BETTER3:92% Commercial40% Part D74% Medicaid Based on~107 MILLION LIVES1 Positive payer response to EPSOLAY®—Competitive pricing likelyequals parity access in rosacea

In January 2018, Perrigo received tentative approval from the FDA for ivermectin cream,1%, developed in
collaboration with Sol-Gel. Perrigo was second to file and, as of today,there is no public disclosure of a third filer to the FDA. In February 2019, Perrigo received approval from the FDA and launched the sale of acyclovir cream,5%, developed
in collaboration with Sol-Gel. An authorized generic product entered the market in the third quarter of 2019. Bioequivalence achieved for generic 5-fluorouracil cream, 5%, for actinic keratosis, submission of abbreviated New Drug Application
expected in 1H 2021. A portfolio of generic product candidates with favorable commercial agreementsthat supplement our branded pipelineSeven collaborations with Perrigo and 1 with Douglas Pharmaceuticalswith 50/50 gross profit sharing FDA
Approvals Recent Developments Multiple Collaborations Revenue-generating generics partnerships

Financial profile Gross proceeds of $86.3 million raised in IPO of 7,187,500 ordinary shares on February
5, 2018Gross proceeds of $11.5 million raised in a public follow-on offering on August 12, 201920,387,468 shares outstanding as ofSeptember 30, 2019$57.7 million of cash and investmentsas of September 30, 2019$18.8 million in generic product
revenue in the first 9 months of 2019 Cash resources expected to be sufficient to fund operational and capital expenditure requirements into the first quarter of 2021

2021 File NDA for EPSOLAY® in 1H/2020 File NDA for TWYENO® in 2H/2020 2019 2020 Obtained ANDA
approval for acyclovir cream (collaboration with Perrigo) Reported positive phase III results for EPSOLAY® in papulopustular rosacea Reported positive phase III results for TWYNEO® in acne vulgaris at end of 2019 Bioequivalence achieved for
generic 5-fluorouracil cream, 5% Recent milestones & next steps US pre-launch commercial preparations File ANDA for 5-fluorouracil cream, 5% in 1H/2021 (collaboration with Douglas) Approval and launch of EPSOLAY® US
commercial organization fully operational TWYNEO® granted market protection out to 2038 Approval and launch of TWYNEO® following EPSOLAY® Recognized non-dilutive revenues early from launch of acyclovir cream (by Perrigo) Initiated
phase I PoC for SGT-210 in palmoplantar keratoderma Top-line data expected in phase I PoC for SGT-210 in 1H/2021

NASDAQ: SLGLwww.sol-gel.com ©2019 Sol-Gel Technologies Ltd. All Rights Reserved. EPSOLAY® is a
registered trademark of Sol-Gel Technologies Ltd. All other trademarks are the property of their respective owners.
